The early isoform of disabled-1 functions independently of Reelin-mediated tyrosine phosphorylation in chick retina
- PMID: 20606009
- PMCID: PMC2937555
- DOI: 10.1128/MCB.00545-10
The early isoform of disabled-1 functions independently of Reelin-mediated tyrosine phosphorylation in chick retina
Abstract
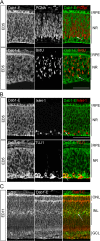
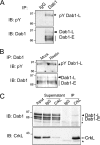
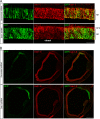
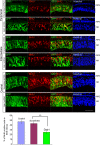
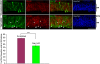
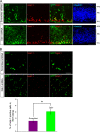
The Reelin-Disabled-1 (Dab1) signaling pathway plays a key role in the positioning of neurons during brain development. Two alternatively spliced Dab1 isoforms have been identified in chick retina and brain: Dab1-E, expressed at early stages of development, and Dab1-L (commonly referred to as Dab1), expressed at later developmental stages. The well-studied Dab1-L serves as an adaptor protein linking Reelin signal to its downstream effectors; however, nothing is known regarding the role of Dab1-E. Here we show that Dab1-E is primarily expressed in proliferating retinal progenitor cells whereas Dab1-L is found exclusively in differentiated neuronal cells. In contrast to Dab1-L, which is tyrosine phosphorylated upon Reelin stimulation, Dab1-E is not tyrosine phosphorylated and may function independently of Reelin. Knockdown of Dab1-E in chick retina results in a significant reduction in the number of proliferating cells and promotes ganglion cell differentiation. Our results demonstrate a role for Dab1-E in the maintenance of the retinal progenitor pool and determination of cell fate.
Figures





Similar articles
-
Serine phosphorylation regulates disabled-1 early isoform turnover independently of Reelin.Cell Signal. 2011 Mar;23(3):555-65. doi: 10.1016/j.cellsig.2010.11.007. Epub 2010 Nov 25. Cell Signal. 2011. PMID: 21111810 Free PMC article.
-
Reelin-Disabled-1 signaling in neuronal migration: splicing takes the stage.Cell Mol Life Sci. 2013 Jul;70(13):2319-29. doi: 10.1007/s00018-012-1171-6. Epub 2012 Sep 28. Cell Mol Life Sci. 2013. PMID: 23052211 Free PMC article. Review.
-
Migration of sympathetic preganglionic neurons in the spinal cord is regulated by Reelin-dependent Dab1 tyrosine phosphorylation and CrkL.J Comp Neurol. 2007 Jun 1;502(4):635-43. doi: 10.1002/cne.21318. J Comp Neurol. 2007. PMID: 17394141
-
Modulation of Reelin signaling by Cyclin-dependent kinase 5.Brain Res. 2007 Apr 6;1140:84-95. doi: 10.1016/j.brainres.2006.01.121. Epub 2006 Mar 10. Brain Res. 2007. PMID: 16529723
-
A missed exit: Reelin sets in motion Dab1 polyubiquitination to put the break on neuronal migration.Genes Dev. 2007 Nov 15;21(22):2850-4. doi: 10.1101/gad.1622907. Genes Dev. 2007. PMID: 18006681 Review. No abstract available.
Cited by
-
Serine phosphorylation regulates disabled-1 early isoform turnover independently of Reelin.Cell Signal. 2011 Mar;23(3):555-65. doi: 10.1016/j.cellsig.2010.11.007. Epub 2010 Nov 25. Cell Signal. 2011. PMID: 21111810 Free PMC article.
-
RBX2 maintains final retinal cell position in a DAB1-dependent and -independent fashion.Development. 2018 Feb 2;145(3):dev155283. doi: 10.1242/dev.155283. Development. 2018. PMID: 29361558 Free PMC article.
-
Reelin-Disabled-1 signaling in neuronal migration: splicing takes the stage.Cell Mol Life Sci. 2013 Jul;70(13):2319-29. doi: 10.1007/s00018-012-1171-6. Epub 2012 Sep 28. Cell Mol Life Sci. 2013. PMID: 23052211 Free PMC article. Review.
-
Disabled 1 Is Part of a Signaling Pathway Activated by Epidermal Growth Factor Receptor.Int J Mol Sci. 2021 Feb 9;22(4):1745. doi: 10.3390/ijms22041745. Int J Mol Sci. 2021. PMID: 33572344 Free PMC article.
-
Linear motifs confer functional diversity onto splice variants.Nucleic Acids Res. 2012 Aug;40(15):7123-31. doi: 10.1093/nar/gks442. Epub 2012 May 25. Nucleic Acids Res. 2012. PMID: 22638587 Free PMC article.
References
-
- Altshuler, D., and C. Cepko. 1992. A temporally regulated, diffusible activity is required for rod photoreceptor development in vitro. Development 114:947-957. - PubMed
-
- Andrade, N., V. Komnenovic, S. M. Blake, Y. Jossin, B. Howell, A. Goffinet, W. J. Schneider, and J. Nimpf. 2007. ApoER2/VLDL receptor and Dab1 in the rostral migratory stream function in postnatal neuronal migration independently of Reelin. Proc. Natl. Acad. Sci. U. S. A. 104:8508-8513. - PMC - PubMed
-
- Arnaud, L., B. A. Ballif, E. Forster, and J. A. Cooper. 2003. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr. Biol. 13:9-17. - PubMed
-
- Austin, C. P., D. E. Feldman, J. A. Ida, Jr., and C. L. Cepko. 1995. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development 121:3637-3650. - PubMed
-
- Ballif, B. A., L. Arnaud, W. T. Arthur, D. Guris, A. Imamoto, and J. A. Cooper. 2004. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr. Biol. 14:606-610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
