Anti-Pseudomonas activity of frog skin antimicrobial peptides in a Caenorhabditis elegans infection model: a plausible mode of action in vitro and in vivo
- PMID: 20606068
- PMCID: PMC2935021
- DOI: 10.1128/AAC.00154-10
Anti-Pseudomonas activity of frog skin antimicrobial peptides in a Caenorhabditis elegans infection model: a plausible mode of action in vitro and in vivo
Abstract
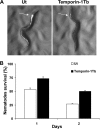
The emergence of multidrug-resistant (MDR) microorganisms makes it increasingly difficult to treat infections. These infections include those associated with Pseudomonas aeruginosa, which are hard to eradicate, especially in patients with a compromised immune system. Naturally occurring membrane-active cationic antimicrobial peptides (CAMPs) serve as attractive candidates for the development of new therapeutic agents. Amphibian skin is one of the richest sources for such peptides, but only a few studies on their in vivo activities and modes of action have been reported. We investigated (i) the activity and mechanism underlying the killing of short CAMPs from frog skin (e.g., temporins and esculentin fragments) on an MDR clinical isolate of P. aeruginosa and (ii) their in vivo antibacterial activities and modes of action, using the minihost model of Caenorhabditis elegans. Our data revealed that in vivo, both temporin-1Tb and esculentin(1-18) were highly active in promoting the survival of Pseudomonas-infected nematodes, although temporin-1Tb did not show significant activity in vitro under the experimental conditions used. Importantly, esculentin(1-18) permeated the membrane of Pseudomonas cells within the infected nematode. To the best of our knowledge, this is the first report showing the ability of a CAMP to permeate the microbial membrane within a living organism. Besides shedding light on a plausible mode of action of frog skin CAMPs in vivo, our data suggest that temporins and esculentins would be attractive molecules as templates for the development of new therapeutics against life-threatening infections.
Figures





References
-
- Ausubel, F. M. 2005. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 6:973-979. - PubMed
-
- Bhavsar, A. P., and E. D. Brown. 2006. The worm turns for antimicrobial discovery. Nat. Biotechnol. 24:1098-1100. - PubMed
-
- Carotenuto, A., S. Malfi, M. R. Saviello, P. Campiglia, I. Gomez-Monterrey, M. L. Mangoni, L. M. Gaddi, E. Novellino, and P. Grieco. 2008. A different molecular mechanism underlying antimicrobial and hemolytic actions of temporins A and L. J. Med. Chem. 51:2354-2362. - PubMed
-
- Chamilos, G., M. S. Lionakis, R. E. Lewis, and D. P. Kontoyiannis. 2007. Role of mini-host models in the study of medically important fungi. Lancet Infect. Dis. 7:42-55. - PubMed
-
- Cirioni, O., C. Silvestri, R. Ghiselli, F. Orlando, A. Riva, F. Mocchegiani, L. Chiodi, S. Castelletti, E. Gabrielli, V. Saba, G. Scalise, and A. Giacometti. 2008. Protective effects of the combination of alpha-helical antimicrobial peptides and rifampicin in three rat models of Pseudomonas aeruginosa infection. J. Antimicrob. Chemother. 62:1332-1338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

