Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart
- PMID: 20606117
- PMCID: PMC2907453
- DOI: 10.1161/CIRCULATIONAHA.110.953208
Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart
Abstract
Background: Elderly patients are more sensitive than younger patients to myocardial ischemia, which results in higher mortality. We investigated how aging affects the cardioprotective AMP-activated protein kinase (AMPK) signaling pathway.
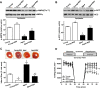
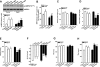
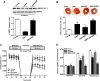
Methods and results: Ischemic AMPK activation was impaired in aged compared with young murine hearts. The expression and secretion of the AMPK upstream regulator, macrophage migration inhibitory factor (MIF), were lower in aged compared with young adult hearts. Additionally, the levels of hypoxia-inducible factor 1alpha, a known transcriptional activator of MIF, were reduced in aged compared with young hearts. Ischemia-induced AMPK activation in MIF knockout mice was blunted, leading to greater contractile dysfunction in MIF-deficient than in wild-type hearts. Furthermore, intramyocardial injection of adenovirus encoding MIF in aged mice increased MIF expression and ischemic AMPK activation and reduced infarct size.
Conclusions: An impaired MIF-AMPK activation response in senescence thus may be attributed to an aging-associated defect in hypoxia-inducible factor 1alpha, the transcription factor for MIF. In the clinical setting, impaired cardiac hypoxia-inducible factor 1alpha activation and consequent reduced MIF expression may play an important role in the increased susceptibility to myocardial ischemia observed in older cardiac patients.
Figures
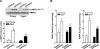






References
-
- Rubino A, Yellon DM. Ischaemic preconditioning of the vasculature: an overlooked phenomenon for protecting the heart? Trends Pharmacol Sci. 2000;21:225–230. - PubMed
-
- Starnes JW, Bowles DK, Seiler KS. Myocardial injury after hypoxia in immature, adult and aged rats. Aging (Milano) 1997;9:268–276. - PubMed
-
- Mariani J, Ou R, Bailey M, Rowland M, Nagley P, Rosenfeldt F, Pepe S. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg. 2000;120:660–667. - PubMed
-
- Abete P, Napoli C, Santoro G, Ferrara N, Tritto I, Chiariello M, Rengo F, Ambrosio G. Age-related decrease in cardiac tolerance to oxidative stress. J Mol Cell Cardiol. 1999;31:227–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

