Human GnRH deficiency: a unique disease model to unravel the ontogeny of GnRH neurons
- PMID: 20606386
- PMCID: PMC3214927
- DOI: 10.1159/000314193
Human GnRH deficiency: a unique disease model to unravel the ontogeny of GnRH neurons
Abstract
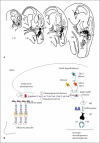
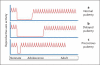
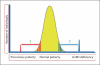
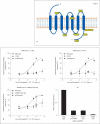
Evolutionary survival of a species is largely a function of its reproductive fitness. In mammals, a sparsely populated and widely dispersed network of hypothalamic neurons, the gonadotropin-releasing hormone (GnRH) neurons, serve as the pilot light of reproduction via coordinated secretion of GnRH. Since it first description, human GnRH deficiency has been recognized both clinically and genetically as a heterogeneous disease. A spectrum of different reproductive phenotypes comprised of congenital GnRH deficiency with anosmia (Kallmann syndrome), congenital GnRH deficiency with normal olfaction (normosmic idiopathic hypogonadotropic hypogonadism), and adult-onset hypogonadotropic hypogonadism has been described. In the last two decades, several genes and pathways which govern GnRH ontogeny have been discovered by studying humans with GnRH deficiency. More importantly, detailed study of these patients has highlighted the emerging theme of oligogenicity and genotypic synergism, and also expanded the phenotypic diversity with the documentation of reversal of GnRH deficiency later in adulthood in some patients. The underlying genetic defect has also helped understand the associated nonreproductive phenotypes seen in some of these patients. These insights now provide practicing clinicians with targeted genetic diagnostic strategies and also impact on clinical management.
Copyright 2010 S. Karger AG, Basel.
Figures




References
-
- Wray S, Hoffman G. A developmental study of the quantitative distribution of LHRH neurons within the central nervous system of postnatal male and female rats. J Comp Neurol. 1986;252:522–531. - PubMed
-
- Merchenthaler I, et al. Gonadotropin-releasing hormone (GnRH) neurons and pathways in the rat brain. Cell Tissue Res. 1984;237:15–29. - PubMed
-
- Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab. 2005;90:3122–3127. - PubMed
-
- Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405. - PubMed
-
- Antunes JL, et al. Luteinizing hormone-releasing hormone in human pituitary blood. J Neurosurg. 1978;49:382–386. - PubMed

