The extracellular matrix glycoprotein tenascin-C is beneficial for spinal cord regeneration
- PMID: 20606643
- PMCID: PMC2951554
- DOI: 10.1038/mt.2010.133
The extracellular matrix glycoprotein tenascin-C is beneficial for spinal cord regeneration
Abstract
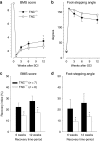
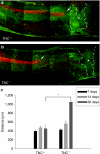
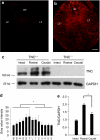
Tenascin-C (TNC), a major component of the extracellular matrix, is strongly upregulated after injuries of the central nervous system (CNS) but its role in tissue repair is not understood. Both regeneration promoting and inhibiting roles of TNC have been proposed considering its abilities to both support and restrict neurite outgrowth in vitro. Here, we show that spontaneous recovery of locomotor functions after spinal cord injury is impaired in adult TNC-deficient (TNC(-/-)) mice in comparison to wild-type (TNC(+/+)) mice. The impaired recovery was associated with attenuated excitability of the plantar Hoffmann reflex (H-reflex), reduced glutamatergic input, reduced sprouting of monaminergic axons in the lumbar spinal cord and enhanced post-traumatic degeneration of corticospinal axons. The degeneration of corticospinal axons in TNC(-/-) mice was normalized to TNC(+/+) levels by application of the alternatively spliced TNC fibronectin type III homologous domain D (fnD). Finally, overexpression of TNC-fnD via adeno-associated virus in wild-type mice improved locomotor recovery, increased monaminergic axons sprouting, and reduced lesion scar volume after spinal cord injury. The functional efficacy of the viral-mediated TNC indicates a potentially useful approach for treatment of spinal cord injury.
Figures
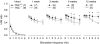



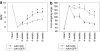
References
-
- GrandPré T, Nakamura F, Vartanian T., and, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. - PubMed
-
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ., and, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. - PubMed
-
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. - PubMed
-
- Niclou SP, Franssen EH, Ehlert EM, Taniguchi M., and, Verhaagen J. Meningeal cell-derived semaphorin 3A inhibits neurite outgrowth. Mol Cell Neurosci. 2003;24:902–912. - PubMed
-
- Jones LL, Margolis RU., and, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

