MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration
- PMID: 20606686
- PMCID: PMC2932764
- DOI: 10.1038/jcbfm.2010.101
MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration
Abstract
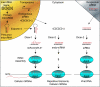
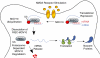
MicroRNAs are small RNAs that function as regulators of posttranscriptional gene expression. MicroRNAs are encoded by genes, and processed to form ribonucleoprotein complexes that bind to messenger RNA (mRNA) targets to repress translation or degrade mRNA transcripts. The microRNAs are particularly abundant in the brain where they serve as effectors of neuronal development and maintenance of the neuronal phenotype. They are also expressed in dendrites where they regulate spine structure and function as effectors in synaptic plasticity. MicroRNAs have been evaluated for their roles in brain ischemia, traumatic brain injury, and spinal cord injury, and in functional recovery after ischemia. They also serve as mediators in the brain's response to ischemic preconditioning that leads to endogenous neuroprotection. In addition, microRNAs are implicated in neurodegenerative disorders, including Alzheimer's, Huntington, Parkinson, and Prion disease. The discovery of microRNAs has expanded the potential for human diseases to arise from genetic mutations in microRNA genes or sequences within their target mRNAs. This review discusses microRNA discovery, biogenesis, mechanisms of gene regulation, their expression and function in the brain, and their roles in brain ischemia and injury, neuroprotection, and neurodegeneration.
Figures

References
-
- Alexiou P, Maragkakis M, Papadopoulos GL, Reczko M, Hatzigeorgiou AG. Lost in translation: an assessment and perspective for computational microRNA target identification. Bioinformatics. 2009;25:3049–3055. - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
-
- Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science. 2007;318:761–764. - PubMed
-
- Banerjee S, Neveu P, Kosik KS. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron. 2009;64:871–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

