The paleobiological record of photosynthesis
- PMID: 20607406
- PMCID: PMC3021713
- DOI: 10.1007/s11120-010-9577-1
The paleobiological record of photosynthesis
Abstract
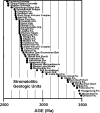
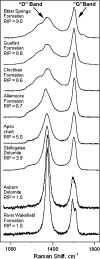
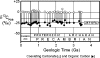
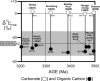
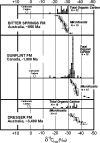
Fossil evidence of photosynthesis, documented in Precambrian sediments by microbially laminated stromatolites, cyanobacterial microscopic fossils, and carbon isotopic data consistent with the presence of Rubisco-mediated CO2-fixation, extends from the present to ~3,500 million years ago. Such data, however, do not resolve time of origin of O2-producing photoautotrophy from its anoxygenic, bacterial, evolutionary precursor. Though it is well established that Earth's ecosystem has been based on autotrophy since its very early stages, the time of origin of oxygenic photosynthesis, more than 2,450 million years ago, has yet to be established.
Figures





Similar articles
-
Geological evidence of oxygenic photosynthesis and the biotic response to the 2400-2200 ma "great oxidation event".Biochemistry (Mosc). 2014 Mar;79(3):165-77. doi: 10.1134/S0006297914030018. Biochemistry (Mosc). 2014. PMID: 24821442 Review.
-
The role of biology in planetary evolution: cyanobacterial primary production in low-oxygen Proterozoic oceans.Environ Microbiol. 2016 Feb;18(2):325-40. doi: 10.1111/1462-2920.13118. Epub 2015 Dec 21. Environ Microbiol. 2016. PMID: 26549614 Free PMC article. Review.
-
Morphological record of oxygenic photosynthesis in conical stromatolites.Proc Natl Acad Sci U S A. 2009 Jul 7;106(27):10939-43. doi: 10.1073/pnas.0900885106. Epub 2009 Jun 29. Proc Natl Acad Sci U S A. 2009. PMID: 19564621 Free PMC article.
-
The initiation of biological processes on Earth: summary of empirical evidence.Adv Space Res. 1992;12(4):143-56. doi: 10.1016/0273-1177(92)90168-w. Adv Space Res. 1992. PMID: 11538134 Review.
-
Earth's early biosphere.Gravit Space Biol Bull. 1998 May;11(2):23-30. Gravit Space Biol Bull. 1998. PMID: 11540635 Review.
Cited by
-
Timing the Evolutionary Advent of Cyanobacteria and the Later Great Oxidation Event Using Gene Phylogenies of a Sunscreen.mBio. 2019 May 21;10(3):e00561-19. doi: 10.1128/mBio.00561-19. mBio. 2019. PMID: 31113897 Free PMC article.
-
The Origin of RNA and the Formose-Ribose-RNA Pathway.Int J Mol Sci. 2024 Jun 19;25(12):6727. doi: 10.3390/ijms25126727. Int J Mol Sci. 2024. PMID: 38928433 Free PMC article. Review.
-
A Comprehensive Study of Cyanobacterial Morphological and Ecological Evolutionary Dynamics through Deep Geologic Time.PLoS One. 2016 Sep 20;11(9):e0162539. doi: 10.1371/journal.pone.0162539. eCollection 2016. PLoS One. 2016. PMID: 27649395 Free PMC article.
-
Thermal-stable proteins of fruit of long-living Sacred Lotus Nelumbo nucifera Gaertn var. China Antique.Trop Plant Biol. 2013 Sep 1;6(2-3):10.1007/s12042-013-9124-2. doi: 10.1007/s12042-013-9124-2. Trop Plant Biol. 2013. PMID: 24363819 Free PMC article.
-
Cyanotoxins and the Nervous System.Toxins (Basel). 2021 Sep 16;13(9):660. doi: 10.3390/toxins13090660. Toxins (Basel). 2021. PMID: 34564664 Free PMC article. Review.
References
-
- Allwood AC, Kamber BS, Walter MR, Burch IW, Kanik I. Trace elements record depositional history of an Early Archean stromatolitic carbonate platform. Chem Geol. 2010;270:148–163. doi: 10.1016/j.chemgeo.2009.11.013. - DOI

