ApoD, a glia-derived apolipoprotein, is required for peripheral nerve functional integrity and a timely response to injury
- PMID: 20607718
- PMCID: PMC7165554
- DOI: 10.1002/glia.21010
ApoD, a glia-derived apolipoprotein, is required for peripheral nerve functional integrity and a timely response to injury
Abstract
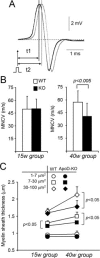
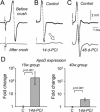
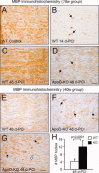
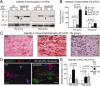
Glial cells are a key element to the process of axonal regeneration, either promoting or inhibiting axonal growth. The study of glial derived factors induced by injury is important to understand the processes that allow or preclude regeneration, and can explain why the PNS has a remarkable ability to regenerate, while the CNS does not. In this work we focus on Apolipoprotein D (ApoD), a Lipocalin expressed by glial cells in the PNS and CNS. ApoD expression is strongly induced upon PNS injury, but its role has not been elucidated. Here we show that ApoD is required for: (1) the maintenance of peripheral nerve function and tissue homeostasis with age, and (2) an adequate and timely response to injury. We study crushed sciatic nerves at two ages using ApoD knock-out and transgenic mice over-expressing human ApoD. The lack of ApoD decreases motor nerve conduction velocity and the thickness of myelin sheath in intact nerves. Following injury, we analyze the functional recovery, the cellular processes, and the protein and mRNA expression profiles of a group of injury-induced genes. ApoD helps to recover locomotor function after injury, promoting myelin clearance, and regulating the extent of angiogenesis and the number of macrophages recruited to the injury site. Axon regeneration and remyelination are delayed without ApoD and stimulated by excess ApoD. The mRNA and protein expression profiles reveal that ApoD is functionally connected in an age-dependent manner to specific molecular programs triggered by injury.
Figures



References
-
- Al‐Shahrour F,Minguez P,Tarraga J,Medina I,Alloza E,Montaner D,Dopazo J. 2007. FatiGO +: A functional profiling tool for genomic data. Integration of functional annotation, regulatory motifs and interaction data with microarray experiments. Nucleic Acids Res 35(Web Server issue): W91–W96. - PMC - PubMed
-
- Balboa MA,Balsinde J. 2006. Oxidative stress and arachidonic acid mobilization. Biochim Biophys Acta 1761: 385–391. - PubMed
-
- Bosse F,Hasenpusch‐Theil K,Kury P,Muller HW. 2006. Gene expression profiling reveals that peripheral nerve regeneration is a consequence of both novel injury‐dependent and reactivated developmental processes. J Neurochem 96: 1441–1457. - PubMed
-
- Boyles JK,Notterpek LM,Anderson LJ. 1990. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A‐IV, apolipoprotein E, apolipoprotein A‐I. J Biol Chem 265: 17805–17815. - PubMed
-
- Cavaggioni A,Pelosi P,Edwards S,Sawyer L. 2006. Functional aspects of β‐lactoglobulin, major urinary protein and odorant binding protein. In: Akerstrom B,Borregaard N,Flower D,Salier J, editors. Lipocalins. Georgetown, Texas: Landes Bioscience. pp 131–139.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

