Agents that bind annexin A2 suppress ocular neovascularization
- PMID: 20607799
- PMCID: PMC4005718
- DOI: 10.1002/jcp.22296
Agents that bind annexin A2 suppress ocular neovascularization
Abstract
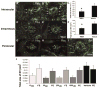
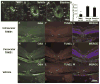
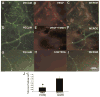
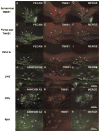
TM601 is a synthetic polypeptide with sequence derived from the venom of the scorpion Leiurus quinquestriatus that has anti-neoplastic activity. It has recently been demonstrated to bind annexin A2 on cultured tumor and vascular endothelial cells and to suppress blood vessel growth on chick chorioallantoic membrane. In this study, we investigated the effects of TM601 in models of ocular neovascularization (NV). When administered by intraocular injection, intravenous injections, or periocular injections, TM601 significantly suppressed the development of choroidal NV at rupture sites in Bruch's membrane. Treatment of established choroidal NV with TM601 caused apoptosis of endothelial cells and regression of the NV. TM601 suppressed ischemia-induced and vascular endothelial growth factor-induced retinal NV and reduced excess vascular permeability induced by vascular endothelial growth factor. Immunostaining with an antibody directed against TM601 showed that after intraocular or periocular injection, TM601 selectively bound to choroidal or retinal NV and co-localized with annexin A2, which is undetectable in normal retinal and choroidal vessels, but is upregulated in endothelial cells participating in choroidal or retinal NV. Intraocular injection of plasminogen or tissue plasminogen activator, which like TM601 bind to annexin A2, also suppressed retinal NV. This study supports the hypothesis that annexin A2 is an important target for treatment of neovascular diseases and suggests that TM601, through its interaction with annexin A2, causes suppression and regression of ocular NV and reduces vascular leakage and thus may provide a new treatment for blinding diseases such as neovascular age-related macular degeneration and diabetic retinopathy.
© 2010 Wiley-Liss, Inc.
Figures


References
-
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S Anchor Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. - PubMed
-
- Campochiaro PA. Ocular versus extraocular neovascularization: Mirror images or vague resemblances. Invest Ophthalmol Vis Sci. 2006;47:462–474. - PubMed
-
- Cesarman GM, Guevara CA, Hajjar KA. An endothelial cell receptor for plasminogen and tissue plasminogen activator. II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J Biol Chem. 1994;269:21198–21203. - PubMed
-
- DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol. 1993;264:C361–C369. - PubMed
-
- Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem. 2004;279:43411–43418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

