Immobilization of iron oxide magnetic nanoparticles for enhancement of vessel wall magnetic resonance imaging--an ex vivo feasibility study
- PMID: 20608720
- PMCID: PMC2923466
- DOI: 10.1021/bc100138c
Immobilization of iron oxide magnetic nanoparticles for enhancement of vessel wall magnetic resonance imaging--an ex vivo feasibility study
Abstract
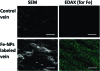
Emerging data supports a role for negative wall remodeling in the failure of vascular interventions such as vein grafts, yet clinicians/researchers currently lack the ability to temporally/efficiently investigate adventitial surface topography/total vascular wall anatomy in vivo. We established a strategy of immobilizing commercially available iron oxide magnetic nanoparticles (Fe-NPs) onto the surface of human vein conduits to facilitate high-throughput total vascular wall demarcation with magnetic resonance (MR). Binding of activated Fe-NPs to amine groups on the surface of the veins induced a thin layer of negative contrast that differentiated the adventitia from surrounding saline signal in all MR images, enabling delineation of total wall anatomy; this was not possible in simultaneously imaged unlabeled control veins. Under the conditions of this ex vivo experiment, stable covalent binding of Fe-NPs can be achieved (dose-dependent) on human vein surface for MR detection, suggesting a potential strategy for enhancing the ability of MRI to investigate total wall adaptation and remodeling in vein graft failure.
Figures






Similar articles
-
Cellular level loading and heating of superparamagnetic iron oxide nanoparticles.Langmuir. 2007 Nov 20;23(24):12329-36. doi: 10.1021/la701100r. Epub 2007 Oct 26. Langmuir. 2007. PMID: 17960940
-
On the use of superparamagnetic hydroxyapatite nanoparticles as an agent for magnetic and nuclear in vivo imaging.Acta Biomater. 2018 Jun;73:458-469. doi: 10.1016/j.actbio.2018.04.040. Epub 2018 Apr 22. Acta Biomater. 2018. PMID: 29689381
-
Glycol chitosan/heparin immobilized iron oxide nanoparticles with a tumor-targeting characteristic for magnetic resonance imaging.Biomacromolecules. 2011 Jun 13;12(6):2335-43. doi: 10.1021/bm200413a. Epub 2011 May 3. Biomacromolecules. 2011. PMID: 21506550
-
Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances.Biotechnol Adv. 2015 Nov 1;33(6 Pt 2):1162-76. doi: 10.1016/j.biotechadv.2015.02.003. Epub 2015 Feb 14. Biotechnol Adv. 2015. PMID: 25689073 Review.
-
Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents.Chem Soc Rev. 2012 Apr 7;41(7):2575-89. doi: 10.1039/c1cs15248c. Epub 2011 Dec 2. Chem Soc Rev. 2012. PMID: 22138852 Review.
Cited by
-
Early animal model evaluation of an implantable contrast agent to enhance magnetic resonance imaging of arterial bypass vein grafts.Acta Radiol. 2018 Sep;59(9):1074-1081. doi: 10.1177/0284185117753656. Epub 2018 Jan 29. Acta Radiol. 2018. PMID: 29378421 Free PMC article.
-
Thirty-day vein remodeling is predictive of midterm graft patency after lower extremity bypass.J Vasc Surg. 2013 Jan;57(1):9-18. doi: 10.1016/j.jvs.2012.06.098. Epub 2012 Sep 7. J Vasc Surg. 2013. PMID: 22960020 Free PMC article.
References
-
- Conte M. S.; Bandyk D. F.; Clowes A. W.; Moneta G. L.; Seely L.; Lorenz T. J.; Namini H.; Hamdan A. D.; Roddy S. P.; Belkin M.; Berceli S. A.; DeMasi R. J.; Samson R. H.; Berman S. S. (2006) Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J. Vasc. Surg. 43, 742–751discussion 751. - PubMed
-
- Alexander J. H.; Hafley G.; Harrington R. A.; Peterson E. D.; Ferguson T. B. Jr.; Lorenz T. J.; Goyal A.; Gibson M.; Mack M. J.; Gennevois D.; Califf R. M.; Kouchoukos N. T. (2005) Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 294, 2446–2454. - PubMed
-
- Lau G. T.; Ridley L. J.; Bannon P. G.; Wong L. A.; Trieu J.; Brieger D. B.; Lowe H. C.; Freedman B. S.; Kritharides L. (2006) Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness. Circulation 114, I435–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

