C-H bonds as ubiquitous functionality: a general approach to complex arylated imidazoles via regioselective sequential arylation of all three C-H bonds and regioselective N-alkylation enabled by SEM-group transposition
- PMID: 20608749
- PMCID: PMC2971672
- DOI: 10.1021/jo100727j
C-H bonds as ubiquitous functionality: a general approach to complex arylated imidazoles via regioselective sequential arylation of all three C-H bonds and regioselective N-alkylation enabled by SEM-group transposition
Abstract
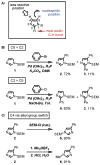
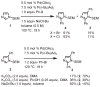
Imidazoles are an important group of the azole family of heterocycles frequently found in pharmaceuticals, drug candidates, ligands for transition metal catalysts, and other molecular functional materials. Owing to their wide application in academia and industry, new methods and strategies for the generation of functionalized imidazole derivatives are in demand. We here describe a general and comprehensive approach for the synthesis of complex aryl imidazoles, where all three C-H bonds of the imidazole core can be arylated in a regioselective and sequential manner. We report new catalytic methods for selective C5- and C2-arylation of SEM-imidazoles and provide a mechanistic hypothesis for the observed positional selectivity based on electronic properties of C-H bonds and the heterocyclic ring. Importantly, aryl bromides and low-cost aryl chlorides can be used as arene donors under practical laboratory conditions. To circumvent the low reactivity of the C-4 position, we developed the SEM-switch that transfers the SEM-group from N-1 to N-3 nitrogen and thus enables preparation of 4-arylimidazoles and sequential C4-C5-arylation of the imidazole core. Furthermore, selective N3-alkylation followed by the SEM-group deprotection (trans-N-alkylation) allows for regioselective N-alkylation of complex imidazoles. The sequential C-arylation enabled by the SEM-switch allowed us to produce a variety of mono-, di-, and triarylimidazoles using diverse bromo- and chloroarenes. Using our approach, the synthesis of individual compounds or libraries of analogues can begin from either the parent imidazole or a substituted imidazole, providing rapid access to complex imidazole structures.
Figures
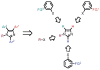


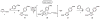
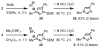
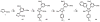
References
-
-
Reviews: Xi N, Huang Q, Liu L. In: Comprehensive Heterocyclic Chemistry. Katritzky AR, Ramsden CA, Scriven EFV, Taylor RJK, editors. Vol. 4. Elsevier; Oxford: 2008. pp. 143–364.Luca LD. Curr Med Chem. 2006;13:1–23.
-
-
-
4,5-Diarylimidazoles showed antibacterial activity. Antolini M, Bozzoli A, Ghiron C, Kennedy G, Rossi T, Ursini A. Bioorg Med Chem Lett. 1999;9:1023–1028.
-
-
-
4,5-Diarylimidazoles were found to have strong antitubulin and cytotoxic activity. Wang L, Woods KW, Li Q, Barr KJ, McCroskey RW, Hannick SM, Gherke L, Credo RB, Hui YH, Marsh K, Warner R, Lee JY, Zielinski-Mozng N, Frost D, Rosenberg SH, Sham HL. J Med Chem. 2002;45:1697–1711.
-
-
-
2,4,5-Triarylimidazoles inhibit mitogen-activated protein kinase activity. Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR. Nature. 1994;372:739–746.
-
-
- Jackson PF, Bullington JL. Curr Top Med Chem. 2002;2:1011–1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

