Proteomic profiling of the epileptic dentate gyrus
- PMID: 20608933
- PMCID: PMC2951482
- DOI: 10.1111/j.1750-3639.2010.00414.x
Proteomic profiling of the epileptic dentate gyrus
Abstract
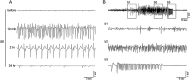
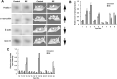
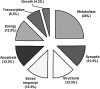
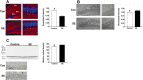
The development of epilepsy is often associated with marked changes in central nervous system cell structure and function. Along these lines, reactive gliosis and granule cell axonal sprouting within the dentate gyrus of the hippocampus are commonly observed in individuals with temporal lobe epilepsy (TLE). Here we used the pilocarpine model of TLE in mice to screen the proteome and phosphoproteome of the dentate gyrus to identify molecular events that are altered as part of the pathogenic process. Using a two-dimensional gel electrophoresis-based approach, followed by liquid chromatography-tandem mass spectrometry, 24 differentially expressed proteins, including 9 phosphoproteins, were identified. Functionally, these proteins were organized into several classes, including synaptic physiology, cell structure, cell stress, metabolism and energetics. The altered expression of three proteins involved in synaptic physiology, actin, profilin 1 and α-synuclein was validated by secondary methods. Interestingly, marked changes in protein expression were detected in the supragranular cell region, an area where robust mossy fibers sprouting occurs. Together, these data provide new molecular insights into the altered protein profile of the epileptogenic dentate gyrus and point to potential pathophysiologic mechanisms underlying epileptogenesis.
© 2010 The Authors; Brain Pathology © 2010 International Society of Neuropathology.
Figures


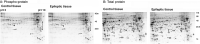
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi‐Lundberg D, Ho WH, Castillo PE et al (2000) Mice lacking alpha‐synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252. - PubMed
-
- Akbar MT, Lundberg AM, Liu K, Vidyadaran S, Wells KE, Dolatshad H et al (2003) The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate‐induced seizures and hippocampal cell death. J Biol Chem 278:19956–19965. - PubMed
-
- Anderson RE, Engstrom FL, Woodbury DM (1984) Localization of carbonic anhydrase in the cerebrum and cerebellum of normal and audiogenic seizure mice. Ann N Y Acad Sci 429:502–504. - PubMed
-
- Birbach A (2008) Profilin, a multi‐modal regulator of neuronal plasticity. Bioessays 30:994–1002. - PubMed

