Bone morphogenetic protein-10 (BMP-10) inhibits aggressiveness of breast cancer cells and correlates with poor prognosis in breast cancer
- PMID: 20608934
- PMCID: PMC11158251
- DOI: 10.1111/j.1349-7006.2010.01648.x
Bone morphogenetic protein-10 (BMP-10) inhibits aggressiveness of breast cancer cells and correlates with poor prognosis in breast cancer
Abstract
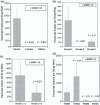
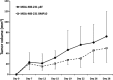
Our recent study showed that a novel member of bone morphogenetic protein (BMP) family, BMP-10, was decreased in prostate cancer. In the present study, we investigated the implication of BMP-10 in breast cancer, particularly the relation of its expression with clinical aspects. The expression of BMP-10 was examined in a cohort of human breast cancer specimens (normal, n = 23; cancer, n = 97), using both quantitative real-time PCR and immunohistochemical staining. The full-length human BMP-10 was cloned into a mammalian expression plasmid vector and then transfected into breast cancer cells. The effect on growth, cell matrix adhesion, motility, and invasion of MDA-MB-231 cells by BMP-10 was then investigated using in vitro growth assays. Immunohistochemical staining and quantitative real-time PCR revealed a decreased expression of BMP-10 in breast cancer. Further analysis of BMP-10 transcript level against the clinical aspect demonstrated that the decreased BMP-10 expression correlated with disease progression, bone metastasis, and poor prognosis. The disease-free survival of the patients with a higher level of BMP-10 was 132.8 (95% CI, 122.0-143.5) months, significantly longer compared to 93.7 (95% CI, 60.3-127.2) months for patients with a lower level of BMP-10 expression (P = 0.043). The overexpression of BMP-10 has broad inhibitory effects on the in vitro growth, invasion, and motility of breast cancer cells. Taken together, BMP-10 can inhibit the cell growth of breast cancer cells, and decreased BMP-10 expression correlates to poor prognosis and disease progression, particularly the lymphatic and bone metastasis. Bone morphogenetic protein-10 (BMP-10) may function as a tumor suppressor in breast cancer.
© 2010 Japanese Cancer Association.
Figures





Similar articles
-
BMP-6 promotes E-cadherin expression through repressing deltaEF1 in breast cancer cells.BMC Cancer. 2007 Nov 13;7:211. doi: 10.1186/1471-2407-7-211. BMC Cancer. 2007. PMID: 17997862 Free PMC article.
-
Bone morphogenetic proteins 1 to 7 in human breast cancer, expression pattern and clinical/prognostic relevance.J Exp Ther Oncol. 2008;7(4):327-38. J Exp Ther Oncol. 2008. PMID: 19227012
-
Clinical implications of the influence of Ehm2 on the aggressiveness of breast cancer cells through regulation of matrix metalloproteinase-9 expression.Mol Cancer Res. 2010 Nov;8(11):1501-12. doi: 10.1158/1541-7786.MCR-10-0186. Epub 2010 Oct 5. Mol Cancer Res. 2010. PMID: 21047774
-
Endogenous bone morphogenetic protein-7 controls the motility of prostate cancer cells through regulation of bone morphogenetic protein antagonists.J Urol. 2007 Sep;178(3 Pt 1):1086-91. doi: 10.1016/j.juro.2007.05.003. Epub 2007 Jul 20. J Urol. 2007. PMID: 17644136
-
Bone morphogenetic proteins in development and progression of breast cancer and therapeutic potential (review).Int J Mol Med. 2009 Nov;24(5):591-7. doi: 10.3892/ijmm_00000269. Int J Mol Med. 2009. PMID: 19787192 Review.
Cited by
-
BMP9, but not BMP10, acts as a quiescence factor on tumor growth, vessel normalization and metastasis in a mouse model of breast cancer.J Exp Clin Cancer Res. 2018 Aug 30;37(1):209. doi: 10.1186/s13046-018-0885-1. J Exp Clin Cancer Res. 2018. PMID: 30165893 Free PMC article.
-
Identification of molecular characteristics induced by radiotherapy in rectal cancer based on microarray data.Oncol Lett. 2017 Apr;13(4):2777-2783. doi: 10.3892/ol.2017.5750. Epub 2017 Feb 20. Oncol Lett. 2017. PMID: 28454466 Free PMC article.
-
BMP10 suppresses hepatocellular carcinoma progression via PTPRS-STAT3 axis.Oncogene. 2019 Nov;38(48):7281-7293. doi: 10.1038/s41388-019-0943-y. Epub 2019 Aug 15. Oncogene. 2019. PMID: 31417183
-
BMP10 inhibited the growth and migration of gastric cancer cells.Tumour Biol. 2016 Mar;37(3):3025-31. doi: 10.1007/s13277-015-4116-5. Epub 2015 Sep 29. Tumour Biol. 2016. PMID: 26419594
-
Deficiency of the adrenomedullin-RAMP3 system suppresses metastasis through the modification of cancer-associated fibroblasts.Oncogene. 2020 Feb;39(9):1914-1930. doi: 10.1038/s41388-019-1112-z. Epub 2019 Nov 21. Oncogene. 2020. PMID: 31754214
References
-
- CancerStats . CancerStats Incidence UK. Cancer Research UK. 2005.
-
- Jemal A, Siegel R, Ward E et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. - PubMed
-
- Ye L, Lewis‐Russell JM, Kyanaston HG, Jiang WG. Bone morphogenetic proteins and their receptor signaling in prostate cancer. Histol Histopathol 2007; 22: 1129–47. - PubMed
-
- Davies SR, Watkins G, Douglas‐Jones A, Mansel RE, Jiang WG. Bone morphogenetic proteins 1 to 7 in human breast cancer, expression pattern and clinical/prognostic relevance. J Exp Ther Oncol 2008; 7: 327–38. - PubMed
-
- Hanavadi S, Martin TA, Watkins G, Mansel RE, Jiang WG. The role of growth differentiation factor‐9 (GDF‐9) and its analog, GDF‐9b/BMP‐15, in human breast cancer. Ann Surg Oncol 2007; 14: 2159–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

