Bone morphogenetic protein-10 (BMP-10) inhibits aggressiveness of breast cancer cells and correlates with poor prognosis in breast cancer
- PMID: 20608934
- PMCID: PMC11158251
- DOI: 10.1111/j.1349-7006.2010.01648.x
Bone morphogenetic protein-10 (BMP-10) inhibits aggressiveness of breast cancer cells and correlates with poor prognosis in breast cancer
Abstract
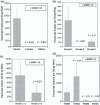
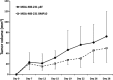
Our recent study showed that a novel member of bone morphogenetic protein (BMP) family, BMP-10, was decreased in prostate cancer. In the present study, we investigated the implication of BMP-10 in breast cancer, particularly the relation of its expression with clinical aspects. The expression of BMP-10 was examined in a cohort of human breast cancer specimens (normal, n = 23; cancer, n = 97), using both quantitative real-time PCR and immunohistochemical staining. The full-length human BMP-10 was cloned into a mammalian expression plasmid vector and then transfected into breast cancer cells. The effect on growth, cell matrix adhesion, motility, and invasion of MDA-MB-231 cells by BMP-10 was then investigated using in vitro growth assays. Immunohistochemical staining and quantitative real-time PCR revealed a decreased expression of BMP-10 in breast cancer. Further analysis of BMP-10 transcript level against the clinical aspect demonstrated that the decreased BMP-10 expression correlated with disease progression, bone metastasis, and poor prognosis. The disease-free survival of the patients with a higher level of BMP-10 was 132.8 (95% CI, 122.0-143.5) months, significantly longer compared to 93.7 (95% CI, 60.3-127.2) months for patients with a lower level of BMP-10 expression (P = 0.043). The overexpression of BMP-10 has broad inhibitory effects on the in vitro growth, invasion, and motility of breast cancer cells. Taken together, BMP-10 can inhibit the cell growth of breast cancer cells, and decreased BMP-10 expression correlates to poor prognosis and disease progression, particularly the lymphatic and bone metastasis. Bone morphogenetic protein-10 (BMP-10) may function as a tumor suppressor in breast cancer.
© 2010 Japanese Cancer Association.
Figures





References
-
- CancerStats . CancerStats Incidence UK. Cancer Research UK. 2005.
-
- Jemal A, Siegel R, Ward E et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. - PubMed
-
- Ye L, Lewis‐Russell JM, Kyanaston HG, Jiang WG. Bone morphogenetic proteins and their receptor signaling in prostate cancer. Histol Histopathol 2007; 22: 1129–47. - PubMed
-
- Davies SR, Watkins G, Douglas‐Jones A, Mansel RE, Jiang WG. Bone morphogenetic proteins 1 to 7 in human breast cancer, expression pattern and clinical/prognostic relevance. J Exp Ther Oncol 2008; 7: 327–38. - PubMed
-
- Hanavadi S, Martin TA, Watkins G, Mansel RE, Jiang WG. The role of growth differentiation factor‐9 (GDF‐9) and its analog, GDF‐9b/BMP‐15, in human breast cancer. Ann Surg Oncol 2007; 14: 2159–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

