Sensory and cortical activation of distinct glial cell subtypes in the somatosensory thalamus of young rats
- PMID: 20608967
- PMCID: PMC2909395
- DOI: 10.1111/j.1460-9568.2010.07281.x
Sensory and cortical activation of distinct glial cell subtypes in the somatosensory thalamus of young rats
Abstract
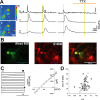
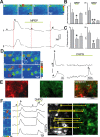
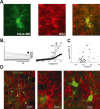
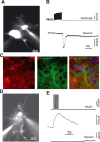
The rodent ventrobasal (VB) thalamus receives sensory inputs from the whiskers and projects to the cortex, from which it receives reciprocal excitatory afferents. Much is known about the properties and functional roles of these glutamatergic inputs to thalamocortical neurons in the VB, but no data are available on how these afferents can affect thalamic glial cells. In this study, we used combined electrophysiological recordings and intracellular calcium ([Ca(2+)](i)) imaging to investigate glial cell responses to synaptic afferent stimulation. VB thalamus glial cells can be divided into two groups based on their [Ca(2+)](i) and electrophysiological responses to sensory and corticothalamic stimulation. One group consists of astrocytes, which stain positively for S100B and preferentially load with SR101, have linear current-voltage relations and low input resistance, show no voltage-dependent [Ca(2+)](i) responses, but express mGluR5-dependent [Ca(2+)](i) transients following stimulation of the sensory and/or corticothalamic excitatory afferent pathways. Cells of the other glial group, by contrast, stain positively for NG2, and are characterized by high input resistance, the presence of voltage-dependent [Ca(2+)](i) elevations and voltage-gated inward currents. There were no synaptically induced [Ca(2+)](i) elevations in these cells under control conditions. These results show that thalamic glial cell responses to synaptic input exhibit different properties to those of thalamocortical neurons. As VB astrocytes can respond to synaptic stimulation and signal to neighbouring neurons, this glial cell organization may have functional implications for the processing of somatosensory information and modulation of behavioural state-dependent thalamocortical network activities.
Figures





Comment in
-
Diversity and specificity of glial cell responses in the thalamus (commentary on Parri et al.).Eur J Neurosci. 2010 Jul;32(1):27-8. doi: 10.1111/j.1460-9568.2010.07328.x. Eur J Neurosci. 2010. PMID: 20608966 No abstract available.
References
-
- Barbaresi P, Spreafico R, Frassoni C, Rustioni A. GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Res. 1986;382:305–326. - PubMed
-
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

