Visfatin is induced by peroxisome proliferator-activated receptor gamma in human macrophages
- PMID: 20608974
- PMCID: PMC3183437
- DOI: 10.1111/j.1742-4658.2010.07729.x
Visfatin is induced by peroxisome proliferator-activated receptor gamma in human macrophages
Abstract
Obesity is a low-grade chronic inflammatory disease associated with an increased number of macrophages (adipose tissue macrophages) in adipose tissue. Within the adipose tissue, adipose tissue macrophages are the major source of visfatin/pre-B-cell colony-enhancing factor/nicotinamide phosphoribosyl transferase. The nuclear receptor peroxisome proliferator-activated receptor gamma (PPARgamma) exerts anti-inflammatory effects in macrophages by inhibiting cytokine production and enhancing alternative differentiation. In this study, we investigated whether PPARgamma modulates visfatin expression in murine (bone marrow-derived macrophage) and human (primary human resting macrophage, classical macrophage, alternative macrophage or adipose tissue macrophage) macrophage models and pre-adipocyte-derived adipocytes. We show that synthetic PPARgamma ligands increase visfatin gene expression in a PPARgamma-dependent manner in primary human resting macrophages and in adipose tissue macrophages, but not in adipocytes. The threefold increase of visfatin mRNA was paralleled by an increase of protein expression (30%) and secretion (30%). Electrophoretic mobility shift assay experiments and transient transfection assays indicated that PPARgamma induces visfatin promoter activity in human macrophages by binding to a DR1-PPARgamma response element. Finally, we show that PPARgamma ligands increase NAD(+) production in primary human macrophages and that this regulation is dampened in the presence of visfatin small interfering RNA or by the visfatin-specific inhibitor FK866. Taken together, our results suggest that PPARgamma regulates the expression of visfatin in macrophages, leading to increased levels of NAD(+).
Figures



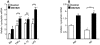
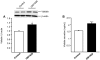
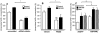
Similar articles
-
Liver X Receptor (LXR) activation negatively regulates visfatin expression in macrophages.Biochem Biophys Res Commun. 2011 Jan 7;404(1):458-62. doi: 10.1016/j.bbrc.2010.12.002. Epub 2010 Dec 8. Biochem Biophys Res Commun. 2011. PMID: 21145308
-
Leukocyte Overexpression of Intracellular NAMPT Attenuates Atherosclerosis by Regulating PPARγ-Dependent Monocyte Differentiation and Function.Arterioscler Thromb Vasc Biol. 2017 Jun;37(6):1157-1167. doi: 10.1161/ATVBAHA.116.308187. Epub 2017 Apr 13. Arterioscler Thromb Vasc Biol. 2017. PMID: 28408371
-
Visfatin/PBEF/Nampt induces EMMPRIN and MMP-9 production in macrophages via the NAMPT-MAPK (p38, ERK1/2)-NF-κB signaling pathway.Int J Mol Med. 2011 Apr;27(4):607-15. doi: 10.3892/ijmm.2011.621. Epub 2011 Feb 15. Int J Mol Med. 2011. PMID: 21327328
-
Review: Peroxisome proliferator-activated receptor gamma and adipose tissue--understanding obesity-related changes in regulation of lipid and glucose metabolism.J Clin Endocrinol Metab. 2007 Feb;92(2):386-95. doi: 10.1210/jc.2006-1268. Epub 2006 Dec 5. J Clin Endocrinol Metab. 2007. PMID: 17148564 Review.
-
An update on visfatin/pre-B cell colony-enhancing factor, an ubiquitously expressed, illusive cytokine that is regulated in obesity.Curr Opin Lipidol. 2006 Apr;17(2):128-31. doi: 10.1097/01.mol.0000217893.77746.4b. Curr Opin Lipidol. 2006. PMID: 16531748 Review.
Cited by
-
Visfatin is regulated by rosiglitazone in type 2 diabetes mellitus and influenced by NFκB and JNK in human abdominal subcutaneous adipocytes.PLoS One. 2011;6(6):e20287. doi: 10.1371/journal.pone.0020287. Epub 2011 Jun 9. PLoS One. 2011. PMID: 21694775 Free PMC article.
-
Visfatin level in children and adolescents with autoimmune thyroiditis.Ther Adv Endocrinol Metab. 2017 Aug;8(8):119-125. doi: 10.1177/2042018817731073. Epub 2017 Sep 25. Ther Adv Endocrinol Metab. 2017. PMID: 28979761 Free PMC article.
-
Genetic Background, Adipocytokines, and Metabolic Disorders in Postmenopausal Overweight and Obese Women.Biochem Genet. 2016 Oct;54(5):636-52. doi: 10.1007/s10528-016-9743-z. Epub 2016 May 31. Biochem Genet. 2016. PMID: 27246401 Free PMC article.
-
Downregulation of the tumour suppressor p16INK4A contributes to the polarisation of human macrophages toward an adipose tissue macrophage (ATM)-like phenotype.Diabetologia. 2011 Dec;54(12):3150-6. doi: 10.1007/s00125-011-2324-0. Epub 2011 Oct 4. Diabetologia. 2011. PMID: 21968977 Free PMC article.
-
Visfatin is involved in TNFα-mediated insulin resistance via an NAD(+)/Sirt1/PTP1B pathway in 3T3-L1 adipocytes.Adipocyte. 2014 Jul 1;3(3):180-9. doi: 10.4161/adip.28729. Epub 2014 Apr 4. Adipocyte. 2014. PMID: 25068084 Free PMC article.
References
-
- Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–34. - PubMed
-
- Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–7. - PubMed
-
- Seo JA, Jang ES, Kim BG, Ryu OH, Kim HY, Lee KW, Kim SG, Choi KM, Baik SH, Choi DS, Kim NH. Plasma visfatin levels are positively associated with circulating interleukin-6 in apparently healthy Korean women. Diabetes Res Clin Pract. 2008;79:108–11. - PubMed

