Transcriptional regulation of human osteopontin promoter by histone deacetylase inhibitor, trichostatin A in cervical cancer cells
- PMID: 20609221
- PMCID: PMC2911447
- DOI: 10.1186/1476-4598-9-178
Transcriptional regulation of human osteopontin promoter by histone deacetylase inhibitor, trichostatin A in cervical cancer cells
Abstract
Background: Trichostatin A (TSA), a potent inhibitor of histone deacetylases exhibits strong anti-tumor and growth inhibitory activities, but its mechanism(s) of action is not completely understood. Osteopontin (OPN) is a secreted glycoprotein which has long been associated with tumor metastasis. Elevated OPN expression in various metastatic cancer cells and the surrounding stromal cells often correlates with enhanced tumor formation and metastasis. To investigate the effects of TSA on OPN transcription, we analyzed a proximal segment of OPN promoter in cervical carcinoma cells.
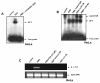
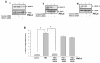
Results: In this paper, we for the first time report that TSA suppresses PMA-induced OPN gene expression in human cervical carcinoma cells and previously unidentified AP-1 transcription factor is involved in this event. Deletion and mutagenesis analyses of OPN promoter led to the characterization of a proximal sequence (-127 to -70) that contain AP-1 binding site. This was further confirmed by gel shift and chromatin immunoprecipitation (ChIP) assays. Western blot and reverse transcription-PCR analyses revealed that TSA suppresses c-jun recruitment to the OPN promoter by inhibiting c-jun levels while c-fos expression was unaffected. Silencing HDAC1 followed by stimulation with PMA resulted in significant decrease in OPN promoter activity suggesting that HDAC1 but not HDAC3 or HDAC4 was required for AP-1-mediated OPN transcription. TSA reduces the PMA-induced hyperacetylation of histones H3 and H4 and recruitment of RNA pol II and TFIIB, components of preinitiation complex to the OPN promoter. The PMA-induced expression of other AP-1 regulated genes like cyclin D1 and uPA was also altered by TSA. Interestingly, PMA promoted cervical tumor growth in mice xenograft model was significantly suppressed by TSA.
Conclusions: In conclusion, these findings provide new insights into mechanisms underlying anticancer activity of TSA and blocking OPN expression at transcriptional level by TSA may act as novel therapeutic strategy for the management of cervical cancer.
Figures





Similar articles
-
Pharmacological Properties of Trichostatin A, Focusing on the Anticancer Potential: A Comprehensive Review.Pharmaceuticals (Basel). 2022 Oct 8;15(10):1235. doi: 10.3390/ph15101235. Pharmaceuticals (Basel). 2022. PMID: 36297347 Free PMC article. Review.
-
Histone deacetylase inhibitors suppress the induction of c-Jun and its target genes including COX-2.J Biol Chem. 2005 Sep 23;280(38):32569-77. doi: 10.1074/jbc.M503201200. Epub 2005 Jul 1. J Biol Chem. 2005. Retraction in: J Biol Chem. 2020 Jan 3;295(1):294. doi: 10.1074/jbc.W119.012139. PMID: 15994313 Retracted.
-
Trichostatin A activates the osteopontin gene promoter through AP1 site.Biochem Biophys Res Commun. 2004 Mar 19;315(4):959-63. doi: 10.1016/j.bbrc.2004.01.152. Biochem Biophys Res Commun. 2004. PMID: 14985105
-
Epigenetic control of HNF-4α in colon carcinoma cells affects MUC4 expression and malignancy.Cell Oncol (Dordr). 2013 Apr;36(2):155-67. doi: 10.1007/s13402-012-0123-3. Epub 2013 Jan 11. Cell Oncol (Dordr). 2013. PMID: 23307400
-
Participation of signaling pathways in the derepression of luteinizing hormone receptor transcription.Mol Cell Endocrinol. 2010 Jan 27;314(2):221-7. doi: 10.1016/j.mce.2009.05.005. Epub 2009 May 21. Mol Cell Endocrinol. 2010. PMID: 19464346 Free PMC article. Review.
Cited by
-
Pharmacological Properties of Trichostatin A, Focusing on the Anticancer Potential: A Comprehensive Review.Pharmaceuticals (Basel). 2022 Oct 8;15(10):1235. doi: 10.3390/ph15101235. Pharmaceuticals (Basel). 2022. PMID: 36297347 Free PMC article. Review.
-
Sp1 and Sp3 Are the Transcription Activators of Human ek1 Promoter in TSA-Treated Human Colon Carcinoma Cells.PLoS One. 2016 Jan 25;11(1):e0147886. doi: 10.1371/journal.pone.0147886. eCollection 2016. PLoS One. 2016. PMID: 26807725 Free PMC article.
-
Osteopontin is a prognostic biomarker in non-small cell lung cancer.BMC Cancer. 2013 Nov 11;13:540. doi: 10.1186/1471-2407-13-540. BMC Cancer. 2013. PMID: 24215488 Free PMC article.
-
Epoxyazadiradione suppresses breast tumor growth through mitochondrial depolarization and caspase-dependent apoptosis by targeting PI3K/Akt pathway.BMC Cancer. 2018 Jan 8;18(1):52. doi: 10.1186/s12885-017-3876-2. BMC Cancer. 2018. PMID: 29310608 Free PMC article.
-
Induction of Osteopontin by Dengue Virus-3 Infection in THP-1 Cells: Inhibition of the Synthesis by Brefelamide and Its Derivative.Front Microbiol. 2017 Mar 29;8:521. doi: 10.3389/fmicb.2017.00521. eCollection 2017. Front Microbiol. 2017. PMID: 28405192 Free PMC article.
References
-
- Craig AM, Bowden GT, Chambers AF, Spearman MA, Greenberg AH, Wright JA, McLeod M, Denhardt DT. Secreted phosphoprotein mRNA is induced during multi-stage carcinogenesis in mouse skin and correlates with the metastatic potential of murine fibroblasts. Int J Cancer. 1990;46:133–137. doi: 10.1002/ijc.2910460124. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

