Murine amniotic fluid stem cells contribute mesenchymal but not epithelial components to reconstituted mammary ducts
- PMID: 20609228
- PMCID: PMC2941112
- DOI: 10.1186/scrt20
Murine amniotic fluid stem cells contribute mesenchymal but not epithelial components to reconstituted mammary ducts
Abstract
Introduction: Amniotic fluid harbors cells indicative of all three germ layers, and pluripotent fetal amniotic fluid stem cells (AFSs) are considered potentially valuable for applications in cellular therapy and tissue engineering. We investigated whether it is possible to direct the cell fate of AFSs in vivo by transplantation experiments into a particular microenvironment, the mammary fat pad. This microenvironment provides the prerequisites to study stem cell function and the communication between mesenchymal and epithelial cells. On clearance of the endogenous epithelium, the ductal tree can be reconstituted by the transfer of exogenously provided mammary stem cells. Analogously, exogenously provided stem cells from other tissues can be investigated for their potential to contribute to mammary gland regeneration.
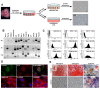
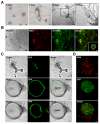
Methods: We derived pluripotent murine AFSs, measured the expression of stem cell markers, and confirmed their in vitro differentiation potential. AFSs were transplanted into cleared and non cleared fat pads of immunocompromised mice to evaluate their ability to assume particular cell fates under the instructive conditions of the fat-pad microenvironment and the hormonal stimulation during pregnancy.
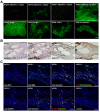
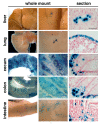
Results: Transplantation of AFSs into cleared fat pads alone or in the presence of exogenous mammary epithelial cells caused their differentiation into stroma and adipocytes and replaced endogenous mesenchymal components surrounding the ducts in co-transplantation experiments. Similarly, transplantation of AFSs into fat pads that had not been previously cleared led to AFS-derived stromal cells surrounding the elongating endogenous ducts. AFSs expressed the marker protein α-SMA, but did not integrate into the myoepithelial cell layer of the ducts in virgin mice. With pregnancy, a small number of AFS-derived cells were present in acinar structures.
Conclusions: Our data demonstrate that the microenvironmental cues of the mammary fat pad cause AFSs to participate in mammary gland regeneration by providing mesenchymal components to emerging glandular structures, but do not incorporate or differentiate into ductal epithelial cells.
Figures

Similar articles
-
A sparing procedure to clear the mouse mammary fat pad of epithelial components for transplantation analysis.Lab Anim. 2008 Jan;42(1):104-10. doi: 10.1258/la.2007.06003e. Lab Anim. 2008. PMID: 18348772 Clinical Trial.
-
Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation.Stem Cells. 2010 May;28(5):928-38. doi: 10.1002/stem.407. Stem Cells. 2010. PMID: 20235097
-
Reconstitution of mammary epithelial morphogenesis by murine embryonic stem cells undergoing hematopoietic stem cell differentiation.PLoS One. 2010 Mar 15;5(3):e9707. doi: 10.1371/journal.pone.0009707. PLoS One. 2010. PMID: 20300573 Free PMC article.
-
Amniotic fluid stem cell-based models to study the effects of gene mutations and toxicants on male germ cell formation.Asian J Androl. 2012 Mar;14(2):247-50. doi: 10.1038/aja.2011.170. Epub 2012 Jan 9. Asian J Androl. 2012. PMID: 22231297 Free PMC article. Review.
-
Murine mammary epithelial stem cells: discovery, function, and current status.Cold Spring Harb Perspect Biol. 2011 Feb 1;3(2):a004879. doi: 10.1101/cshperspect.a004879. Cold Spring Harb Perspect Biol. 2011. PMID: 20926515 Free PMC article. Review.
Cited by
-
Amniotic fluid stem cells with low γ-interferon response showed behavioral improvement in Parkinsonism rat model.PLoS One. 2013 Sep 30;8(9):e76118. doi: 10.1371/journal.pone.0076118. eCollection 2013. PLoS One. 2013. PMID: 24098771 Free PMC article.
-
Perinatal sources of mesenchymal stem cells: Wharton's jelly, amnion and chorion.Cell Mol Biol Lett. 2011 Sep;16(3):493-514. doi: 10.2478/s11658-011-0019-7. Epub 2011 Jul 18. Cell Mol Biol Lett. 2011. PMID: 21786036 Free PMC article. Review.
-
First steps to define murine amniotic fluid stem cell microenvironment.Sci Rep. 2016 Nov 15;6:37080. doi: 10.1038/srep37080. Sci Rep. 2016. PMID: 27845396 Free PMC article.
-
Amniotic fluid as a source of engraftable stem cells.Brain Circ. 2017 Jul-Sep;3(3):175-179. doi: 10.4103/bc.bc_24_17. Epub 2017 Oct 12. Brain Circ. 2017. PMID: 30276321 Free PMC article. Review.
-
Engineering epithelial-stromal interactions in vitro for toxicology assessment.Toxicology. 2017 May 1;382:93-107. doi: 10.1016/j.tox.2017.03.007. Epub 2017 Mar 8. Toxicology. 2017. PMID: 28285100 Free PMC article. Review.
References
-
- Gosden CM. Amniotic fluid cell types and culture. Br Med Bull. 1983;39:348–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

