Probing the probes: fitness factors for small molecule tools
- PMID: 20609406
- PMCID: PMC2905514
- DOI: 10.1016/j.chembiol.2010.05.013
Probing the probes: fitness factors for small molecule tools
Abstract
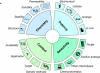
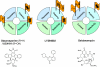
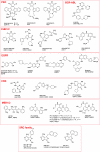
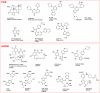
Chemical probes for interrogating biological processes are of considerable current interest. Cell permeable small molecule tools have a major role in facilitating the functional annotation of the human genome, understanding both physiological and pathological processes, and validating new molecular targets. To be valuable, chemical tools must satisfy necessary criteria and recent publications have suggested objective guidelines for what makes a useful chemical probe. Although recognizing that such guidelines may be valuable, we caution against overly restrictive rules that may stifle innovation in favor of a "fit-for-purpose" approach. Reviewing the literature and providing examples from the cancer field, we recommend a series of "fitness factors" to be considered when assessing chemical probes. We hope this will encourage innovative chemical biology research while minimizing the generation of poor quality and misleading biological data, thus increasing understanding of the particular biological area, to the benefit of basic research and drug discovery.
Figures


Similar articles
-
Drugging the PI3 kinome: from chemical tools to drugs in the clinic.Cancer Res. 2010 Mar 15;70(6):2146-57. doi: 10.1158/0008-5472.CAN-09-4355. Epub 2010 Feb 23. Cancer Res. 2010. PMID: 20179189 Free PMC article. Review.
-
Novel Miniaturized Drug Conjugate Leverages HSP90-driven Tumor Accumulation to Overcome PI3K Inhibitor Delivery Challenges to Solid Tumors.Mol Cancer Ther. 2020 Aug;19(8):1613-1622. doi: 10.1158/1535-7163.MCT-19-0964. Epub 2020 Jun 4. Mol Cancer Ther. 2020. PMID: 32499300
-
Probing cancer with small-molecule tools-Progress and challenges.Cancer Cell. 2025 Mar 10;43(3):323-327. doi: 10.1016/j.ccell.2025.02.003. Epub 2025 Feb 27. Cancer Cell. 2025. PMID: 40020670 Review.
-
Chemical Probes for Understudied Kinases: Challenges and Opportunities.J Med Chem. 2022 Jan 27;65(2):1132-1170. doi: 10.1021/acs.jmedchem.1c00980. Epub 2021 Sep 3. J Med Chem. 2022. PMID: 34477374 Review.
-
Downfalls of Chemical Probes Acting at the Kinase ATP-Site: CK2 as a Case Study.Molecules. 2021 Mar 31;26(7):1977. doi: 10.3390/molecules26071977. Molecules. 2021. PMID: 33807474 Free PMC article. Review.
Cited by
-
Small molecule probes for targeting autophagy.Nat Chem Biol. 2021 Jun;17(6):653-664. doi: 10.1038/s41589-021-00768-9. Epub 2021 May 25. Nat Chem Biol. 2021. PMID: 34035513 Review.
-
Chemical Probes Review: Choosing the Right Path Towards Pharmacological Targets in Drug Discovery, Challenges and Future Perspectives.Comb Chem High Throughput Screen. 2024;27(17):2544-2564. doi: 10.2174/0113862073283304231118155730. Comb Chem High Throughput Screen. 2024. PMID: 38083882 Review.
-
Chirality: a key parameter in chemical probes.RSC Chem Biol. 2023 Aug 8;4(10):716-721. doi: 10.1039/d3cb00082f. eCollection 2023 Oct 4. RSC Chem Biol. 2023. PMID: 37799583 Free PMC article.
-
MGMT-independent temozolomide resistance in pediatric glioblastoma cells associated with a PI3-kinase-mediated HOX/stem cell gene signature.Cancer Res. 2010 Nov 15;70(22):9243-52. doi: 10.1158/0008-5472.CAN-10-1250. Epub 2010 Oct 8. Cancer Res. 2010. PMID: 20935218 Free PMC article.
-
Rational methods for the selection of diverse screening compounds.ACS Chem Biol. 2011 Mar 18;6(3):208-17. doi: 10.1021/cb100420r. Epub 2011 Feb 15. ACS Chem Biol. 2011. PMID: 21261294 Free PMC article. Review.
References
-
- Akinaga S., Sugiyama K., Akiyama T. Anticancer Drug Des. 2000;15:43–52. - PubMed
-
- Amidon G.L., Lennernäs H., Shah V.P., Crison J.R. Pharm. Res. 1995;12:413–420. - PubMed
-
- . Come together. Nat. Chem. Biol. 2009;5:863.
-
- . Retooling chemical probes. Nat. Chem. Biol. 2010;6:157. - PubMed
-
- Austin C.P. Curr. Opin. Chem. Biol. 2003;7:511–515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

