Synthesis, quantification, characterization, and signaling properties of glutathionyl conjugates of enals
- PMID: 20609918
- PMCID: PMC3049297
- DOI: 10.1016/S0076-6879(10)74018-0
Synthesis, quantification, characterization, and signaling properties of glutathionyl conjugates of enals
Abstract
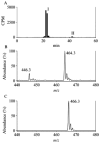
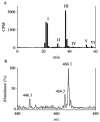
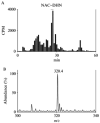
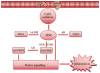
Oxidation of lipids generates large quantities of highly reactive alpha,beta-unsaturated aldehydes (enals). Enals and their protein adducts accumulate in the tissues of several pathologies. In vitro, low concentrations of enals such as HNE (4-hydroxy trans-2-nonenal) affect cell signaling whereas high concentrations of enals are cytotoxic. Direct conjugation of the C2-C3 double bond of enals with the sulfhydryl group of GSH is a major route for the metabolism and detoxification of enals. Recently, we found that glutathionyl conjugate of HNE (GS-HNE) enhances the peritoneal leukocyte infiltration and stimulates the formation of proinflammatory lipid mediators. Moreover, the reduced form of the glutathione conjugate of HNE (GS-DHN) elicits strong mitogenic signaling in smooth muscle cells. In this chapter we discuss the methods to study the metabolism of enals and the redox signaling properties of glutathionyl conjugates of HNE.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth.J Biol Chem. 2006 Jun 30;281(26):17652-60. doi: 10.1074/jbc.M600270200. Epub 2006 Apr 28. J Biol Chem. 2006. PMID: 16648138
-
Identification of biochemical pathways for the metabolism of oxidized low-density lipoprotein derived aldehyde-4-hydroxy trans-2-nonenal in vascular smooth muscle cells.Atherosclerosis. 2001 Oct;158(2):339-50. doi: 10.1016/s0021-9150(01)00454-3. Atherosclerosis. 2001. PMID: 11583712 Free PMC article.
-
Detoxification of cytotoxic alpha,beta-unsaturated aldehydes by carnosine: characterization of conjugated adducts by electrospray ionization tandem mass spectrometry and detection by liquid chromatography/mass spectrometry in rat skeletal muscle.J Mass Spectrom. 2002 Dec;37(12):1219-28. doi: 10.1002/jms.381. J Mass Spectrom. 2002. PMID: 12489081
-
Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease.Redox Biol. 2013 May 23;1(1):319-31. doi: 10.1016/j.redox.2013.04.001. Redox Biol. 2013. PMID: 24024167 Free PMC article. Review.
-
Endogenous glutathione adducts.Curr Drug Metab. 2006 Dec;7(8):853-72. doi: 10.2174/138920006779010601. Curr Drug Metab. 2006. PMID: 17168687 Review.
Cited by
-
Aldose Reductase Differential Inhibitors in Green Tea.Biomolecules. 2020 Jul 6;10(7):1003. doi: 10.3390/biom10071003. Biomolecules. 2020. PMID: 32640594 Free PMC article.
-
Aldehyde reduction by cytochrome P450.Curr Protoc Toxicol. 2011 May;Chapter 4:Unit4.37. doi: 10.1002/0471140856.tx0437s48. Curr Protoc Toxicol. 2011. PMID: 21553396 Free PMC article.
-
Glutathionylated lipid aldehydes are products of adipocyte oxidative stress and activators of macrophage inflammation.Diabetes. 2014 Jan;63(1):89-100. doi: 10.2337/db13-0777. Epub 2013 Sep 23. Diabetes. 2014. PMID: 24062247 Free PMC article.
-
Intra-site differential inhibition of multi-specific enzymes.J Enzyme Inhib Med Chem. 2020 Dec;35(1):840-846. doi: 10.1080/14756366.2020.1743988. J Enzyme Inhib Med Chem. 2020. PMID: 32208768 Free PMC article. Review.
-
Response of a Human Lens Epithelial Cell Line to Hyperglycemic and Oxidative Stress: The Role of Aldose Reductase.Antioxidants (Basel). 2023 Mar 28;12(4):829. doi: 10.3390/antiox12040829. Antioxidants (Basel). 2023. PMID: 37107204 Free PMC article.
References
-
- Adams JD, Jr, Klaidman LK. Acrolein-induced oxygen radical formation. Free Radic Biol Med. 1993;15:187–193. - PubMed
-
- Alary J, Debrauwer L, Fernandez Y, Cravedi JP, Rao D, Bories G. 1,4-Dihydroxynonene mercapturic acid, the major end metabolite of exogenous 4-hydroxy-2-nonenal, is a physiological component of rat and human urine. Chem Res Toxicol. 1998;11:130–135. - PubMed
-
- Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980;620:281–296. - PubMed
-
- Bhatnagar A. Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR024489/RR/NCRR NIH HHS/United States
- R01 HL059378/HL/NHLBI NIH HHS/United States
- GM71036/GM/NIGMS NIH HHS/United States
- R01 HL095593/HL/NHLBI NIH HHS/United States
- HL95593/HL/NHLBI NIH HHS/United States
- R01 ES017260/ES/NIEHS NIH HHS/United States
- P01 ES011860/ES/NIEHS NIH HHS/United States
- R01 ES011594/ES/NIEHS NIH HHS/United States
- R37 DK036118/DK/NIDDK NIH HHS/United States
- Z01 DK036118/ImNIH/Intramural NIH HHS/United States
- ES17260/ES/NIEHS NIH HHS/United States
- HL59378/HL/NHLBI NIH HHS/United States
- ES11860/ES/NIEHS NIH HHS/United States
- RR 24489/RR/NCRR NIH HHS/United States
- R01 DK036118/DK/NIDDK NIH HHS/United States
- R01 GM071036/GM/NIGMS NIH HHS/United States
- ES11594/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

