Role of transmembrane domain 8 in substrate selectivity and translocation of SteT, a member of the L-amino acid transporter (LAT) family
- PMID: 20610400
- PMCID: PMC2937904
- DOI: 10.1074/jbc.M110.116632
Role of transmembrane domain 8 in substrate selectivity and translocation of SteT, a member of the L-amino acid transporter (LAT) family
Abstract
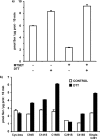
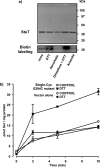
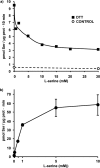
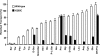
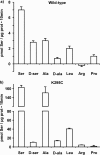
System l-amino acid transporters (LAT) belong to the amino acid, polyamine, and organic cation superfamily of transporters and include the light subunits of heteromeric amino acid transporters and prokaryotic homologues. Cysteine reactivity of SteT (serine/threonine antiporter) has been used here to study the substrate-binding site of LAT transporters. Residue Cys-291, in transmembrane domain 8 (TM8), is inactivated by thiol reagents in a substrate protectable manner. Surprisingly, DTT activated the transporter by reducing residue Cys-291. Cysteine-scanning mutagenesis of TM8 showed DTT activation in the single-cysteine mutants S287C, G294C, and S298C, lining the same alpha-helical face. S-Thiolation in Escherichia coli cells resulted in complete inactivation of the single-cysteine mutant G294C. l-Serine blocked DTT activation with an EC(50) similar to the apparent K(M) of this mutant. Thus, S-thiolation abolished substrate translocation but not substrate binding. Mutation of Lys-295, to Cys (K295C) broadened the profile of inhibitors and the spectrum of substrates with the exception of imino acids. A structural model of SteT based on the structural homologue AdiC (arginine/agmatine antiporter) positions residues Cys-291 and Lys-295 in the putative substrate binding pocket. All this suggests that Lys-295 is a main determinant in the recognition of the side chain of SteT substrates. In contrast, Gly-294 is not facing the surface, suggesting conformational changes involving TM8 during the transport cycle. Our results suggest that TM8 sculpts the substrate-binding site and undergoes conformational changes during the transport cycle of SteT.
Figures



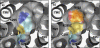
Similar articles
-
Stabilization of a prokaryotic LAT transporter by random mutagenesis.J Gen Physiol. 2016 Apr;147(4):353-68. doi: 10.1085/jgp.201511510. Epub 2016 Mar 14. J Gen Physiol. 2016. PMID: 26976827 Free PMC article.
-
Functional and structural characterization of the first prokaryotic member of the L-amino acid transporter (LAT) family: a model for APC transporters.J Biol Chem. 2007 May 4;282(18):13270-81. doi: 10.1074/jbc.M610695200. Epub 2007 Mar 6. J Biol Chem. 2007. PMID: 17344220
-
Split GFP Complementation as Reporter of Membrane Protein Expression and Stability in E. coli: A Tool to Engineer Stability in a LAT Transporter.Methods Mol Biol. 2017;1586:181-195. doi: 10.1007/978-1-4939-6887-9_11. Methods Mol Biol. 2017. PMID: 28470605
-
Precious things come in little packages.J Mol Microbiol Biotechnol. 2001 Apr;3(2):155-62. J Mol Microbiol Biotechnol. 2001. PMID: 11321568 Review.
-
Structure-function relationships of heterodimeric amino acid transporters.Cell Biochem Biophys. 2002;36(2-3):155-68. doi: 10.1385/CBB:36:2-3:155. Cell Biochem Biophys. 2002. PMID: 12139401 Review.
Cited by
-
Stabilization of a prokaryotic LAT transporter by random mutagenesis.J Gen Physiol. 2016 Apr;147(4):353-68. doi: 10.1085/jgp.201511510. Epub 2016 Mar 14. J Gen Physiol. 2016. PMID: 26976827 Free PMC article.
-
Rush Hour of LATs towards Their Transport Cycle.Membranes (Basel). 2021 Aug 8;11(8):602. doi: 10.3390/membranes11080602. Membranes (Basel). 2021. PMID: 34436365 Free PMC article. Review.
-
Structural bases for the interaction and stabilization of the human amino acid transporter LAT2 with its ancillary protein 4F2hc.Proc Natl Acad Sci U S A. 2014 Feb 25;111(8):2966-71. doi: 10.1073/pnas.1323779111. Epub 2014 Feb 10. Proc Natl Acad Sci U S A. 2014. PMID: 24516142 Free PMC article.
References
-
- Palacín M., Kanai Y. (2004) Pflugers Arch. 447, 490–494 - PubMed
-
- Verrey F., Closs E. I., Wagner C. A., Palacin M., Endou H., Kanai Y. (2004) Pflugers Arch. 447, 532–542 - PubMed
-
- Palacín M., Nunes V., Font-Llitjós M., Jiménez-Vidal M., Fort J., Gasol E., Pineda M., Feliubadaló L., Chillarón J., Zorzano A. (2005) Physiology 20, 112–124 - PubMed
-
- Mastroberardino L., Spindler B., Pfeiffer R., Skelly P. J., Loffing J., Shoemaker C. B., Verrey F. (1998) Nature 395, 288–291 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

