Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation
- PMID: 20610415
- PMCID: PMC2972685
- DOI: 10.1093/cvr/cvq218
Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation
Abstract
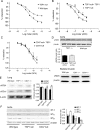
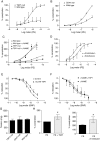
Aims: Thrombospondin-1 (TSP1), via its necessary receptor CD47, inhibits nitric oxide (NO)-stimulated soluble guanylate cyclase activation in vascular smooth muscle cells, and TSP1-null mice have increased shear-dependent blood flow compared with wild-type mice. Yet, the endothelial basement membrane should in theory function as a barrier to diffusion of soluble TSP1 into the arterial smooth muscle cell layer. These findings suggested that endothelial-dependent differences in blood flow in TSP1-null mice may be the result of direct modulation of endothelial NO synthase (eNOS) activation by circulating TSP1. Here we tested the hypothesis that TSP1 inhibits eNOS activation and endothelial-dependent arterial relaxation.
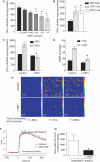
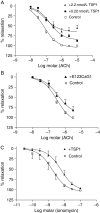
Methods and results: Acetylcholine (ACh)-stimulated activation of eNOS and agonist-driven calcium transients in endothelial cells were inhibited by TSP1. TSP1 also inhibited eNOS phosphorylation at serine(1177). TSP1 treatment of the endothelium of wild-type and TSP1-null but not CD47-null arteries inhibited ACh-stimulated relaxation. TSP1-null vessels demonstrated greater endothelial-dependent vasorelaxation compared with the wild type. Conversely, TSP1-null arteries demonstrated less vasoconstriction to phenylephrine compared with the wild type, which was corrected upon inhibition of eNOS. In TSP1-null mice, intravenous TSP1 blocked ACh-stimulated decreases in blood pressure, and both intravenous TSP1 and a CD47 agonist antibody acutely elevated blood pressure in mice.
Conclusion: TSP1, via CD47, inhibits eNOS activation and endothelial-dependent arterial relaxation and limits ACh-driven decreases in blood pressure. Conversely, intravenous TSP1 and a CD47 antibody increase blood pressure. These findings suggest that circulating TSP1, by limiting endogenous NO production, functions as a pressor agent supporting blood pressure.
Figures


Comment in
-
Thrombospondin-1, endothelium and systemic vascular tone.Future Cardiol. 2011 Mar;7(2):169-72. doi: 10.2217/fca.11.7. Future Cardiol. 2011. PMID: 21453023
References
-
- Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi:10.1016/j.cardiores.2007.03.023. - DOI - PMC - PubMed
-
- Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi:10.1146/annurev.pharmtox.44.101802.121844. - DOI - PubMed
-
- Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–II33. - PubMed
-
- Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi:10.1074/jbc.M000753200. - DOI - PubMed
-
- Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA. 2005;102:13141–13146. doi:10.1073/pnas.0502977102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

