Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development
- PMID: 20610626
- PMCID: PMC2905495
- DOI: 10.1158/0008-5472.CAN-09-4442
Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development
Abstract
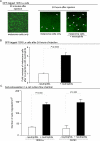
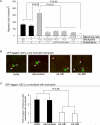
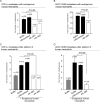
It is unknown why only a minority of circulating tumor cells trapped in lung capillaries form metastases and involvement of immune cells remains uncertain. A novel model has been developed in this study showing that neutrophils regulate lung metastasis development through physical interaction and anchoring of circulating tumor cells to endothelium. Human melanoma cells were i.v. injected into nude mice leading to the entrapment of many cancer cells; however, 24 hours later, very few remained in the lungs. In contrast, injection of human neutrophils an hour after tumor cell injection increased cancer cell retention by approximately 3-fold. Entrapped melanoma cells produced and secreted high levels of a cytokine called interleukin-8 (IL-8), attracting neutrophils and increasing tethering beta(2) integrin expression by 75% to 100%. Intercellular adhesion molecule-1 on melanoma cells and beta(2) integrin on neutrophils interacted, promoting anchoring to vascular endothelium. Decreasing IL-8 secretion from melanoma cells lowered extracellular levels by 20% to 50%, decreased beta(2) integrin on neutrophils by approximately 50%, and reduced neutrophil-mediated extravasation by 25% to 60%, resulting in approximately 50% fewer melanoma cells being tethered to endothelium and retained in lungs. Thus, transendothelial migration and lung metastasis development decreased by approximately 50%, showing that targeting IL-8 in melanoma cells has the potential to decrease metastasis development by disrupting interaction with neutrophils.
(c)2010 AACR.
Figures



References
-
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. - PubMed
-
- Shevde LA, Welch DR. Metastasis suppressor pathways--an evolving paradigm. Cancer Lett. 2003;198:1–20. - PubMed
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

