Interplay of palmitoylation and phosphorylation in the trafficking and localization of phosphodiesterase 10A: implications for the treatment of schizophrenia
- PMID: 20610737
- PMCID: PMC6632485
- DOI: 10.1523/JNEUROSCI.1635-10.2010
Interplay of palmitoylation and phosphorylation in the trafficking and localization of phosphodiesterase 10A: implications for the treatment of schizophrenia
Abstract
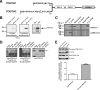
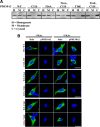
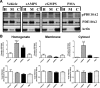
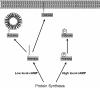
Phosphodiesterase 10A (PDE10A) is a striatum-enriched, dual-specific cyclic nucleotide phosphodiesterase that has gained considerable attention as a potential therapeutic target for psychiatric disorders such as schizophrenia. As such, a PDE10A-selective inhibitor compound, MP-10, has recently entered clinical testing. Since little is known about the cellular regulation of PDE10A, we sought to elucidate the mechanisms that govern its subcellular localization in striatal medium spiny neurons. Previous reports suggest that PDE10A is primarily membrane bound and is transported throughout medium spiny neuron axons and dendrites. Moreover, it has been shown in PC12 cells that the localization of the major splice form, PDE10A2, may be regulated by protein kinase A phosphorylation at threonine 16 (Thr-16). Using an antibody that specifically recognizes phosphorylated Thr-16 (pThr-16) of PDE10A2, we provide evidence that phosphorylation at Thr-16 is critical for the regulation of PDE10A subcellular localization in vivo. Furthermore, we demonstrate in primary mouse striatal neuron cultures that PDE10A membrane association and transport throughout dendritic processes requires palmitoylation of cysteine 11 (Cys-11) of PDE10A2, likely by the palmitoyl acyltransferases DHHC-7 and -19. Finally, we show that Thr-16 phosphorylation regulates PDE10A trafficking and localization by preventing palmitoylation of Cys-11 rather than by interfering with palmitate-lipid interactions. These data support a model whereby PDE10A trafficking and localization can be regulated in response to local fluctuations in cAMP levels. Given this, we propose that excessive striatal dopamine release, as occurs in schizophrenia, might exert differential effects on the regulation of PDE10A localization in the two striatal output pathways.
Figures





References
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42.
-
- Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. - PubMed
-
- Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 2006;21:430–439. - PubMed
-
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. - PubMed
-
- Coskran TM, Morton D, Menniti FS, Adamowicz WO, Kleiman RJ, Ryan AM, Strick CA, Schmidt CJ, Stephenson DT. Immunohistochemical localization of phosphodiesterase 10A in multiple mammalian species. J Histochem Cytochem. 2006;54:1205–1213. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
