Neuronal correlates of memory formation in motor cortex after adaptation to force field
- PMID: 20610753
- PMCID: PMC6632465
- DOI: 10.1523/JNEUROSCI.1603-10.2010
Neuronal correlates of memory formation in motor cortex after adaptation to force field
Abstract
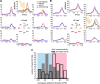
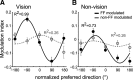
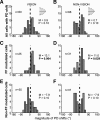
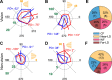
Activity of single neurons in the motor cortex has been shown to change during acquisition of motor skills. We previously reported that the combined activity of cell ensembles in the motor cortex of monkeys (Macaca fascicularis) evolves during adaptation to a novel force field perturbation to encode the direction of compensatory force when reaching to visual targets. We also showed that the population directional signal was altered by the available sensory feedback. Here, we examined whether traces of such activity would linger on to later constitute motor memories of the newly acquired skill and whether memory traces would differ depending on feedback. We found that motor-cortical cell ensembles retained features of their adaptive activity pattern in the absence of perturbation when reaching to both learned and unlearned targets. Moreover, the preferred directions of these cells rotated in the direction of force field while the entire population of cells produced no net rotation of preferred direction when returning to null-field reaches. Whereas the activity pattern and preferred direction rotations were comparable with and without visual feedback, changes in tuning amplitudes differed across feedback conditions. Last, savings in behavioral performance and neuronal activity during later reexposure to force field were apparent. Overall, the findings reflect a novel representation of motor memory by cell ensembles and indicate a putative role of the motor cortex in early acquisition of motor memory.
Figures



References
-
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002;34:1011–1020. - PubMed
-
- Baraduc P, Lang N, Rothwell JC, Wolpert DM. Consolidation of dynamic motor learning is not disrupted by rTMS of primary motor cortex. Curr Biol. 2004;14:252–256. - PubMed
-
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
