Active roles of electrically coupled bipolar cell network in the adult retina
- PMID: 20610761
- PMCID: PMC6632464
- DOI: 10.1523/JNEUROSCI.1590-10.2010
Active roles of electrically coupled bipolar cell network in the adult retina
Abstract
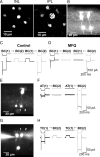
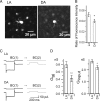
Gap junctions are frequently observed in the adult vertebrate retina. It has been shown that gap junctions function as passive electrotonic pathways and play various roles, such as noise reduction, synchronization of electrical activities, regulation of the receptive field size, and transmission of rod signals to cone pathways. The presence of gap junctions between bipolar cells has been reported in various species but their functions are not known. In the present study, we applied dual whole-cell clamp techniques to the adult goldfish retina to elucidate the functions of gap junctions between ON-type bipolar cells with a giant axon terminal (Mb1-BCs). Electrophysiological and immunohistochemical experiments revealed that Mb1-BCs were coupled with each other through gap junctions that were located at the distal dendrites. The coupling conductance between Mb1-BCs under light-adapted conditions was larger than that under dark-adapted conditions. The gap junctions showed neither rectification nor voltage dependence, and behaved as a low-pass filter. Mb1-BCs could generate Ca(2+) spikes in response to depolarization, especially under dark-adapted conditions. The Ca(2+) spike evoked electrotonic depolarization through gap junctions in neighboring Mb1-BCs, and the depolarization in turn could trigger Ca(2+) spikes with a time lag. A brief depolarizing pulse applied to an Mb1-BC evoked a long-lasting EPSC in the postsynaptic ganglion cell. The EPSC was shortened in duration when gap junctions were pharmacologically or mechanically impaired. These results suggest that the spread of Ca(2+) spikes through gap junctions between bipolar cells may play a key role in lateral interactions in the adult retina.
Figures




References
-
- Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Höher T, Matsubara M, Willecke K, Spray DC, Dermietzel R. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci U S A. 2008;105:20964–20969. - PMC - PubMed
-
- Arai I, Yamada Y, Asaka T, Tachibana M. Light-evoked oscillatory discharges in retinal ganglion cells are generated by rhythmic synaptic inputs. J Neurophysiol. 2004;92:715–725. - PubMed
-
- Bloomfield SA, Xin D, Persky SE. A comparison of receptive field and tracer coupling size of horizontal cells in the rabbit retina. Vis Neurosci. 1995;12:985–999. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
