Increased cyclooxygenase-2 expression and prostaglandin E2 production in pressurized renal medullary interstitial cells
- PMID: 20610829
- PMCID: PMC2944419
- DOI: 10.1152/ajpregu.00544.2009
Increased cyclooxygenase-2 expression and prostaglandin E2 production in pressurized renal medullary interstitial cells
Abstract
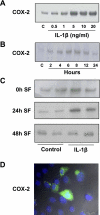
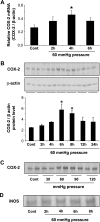
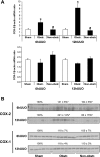
Renal medullary interstitial cells (RMICs) are subjected to osmotic, inflammatory, and mechanical stress as a result of ureteral obstruction, which may influence the expression and activity of cyclooxygenase type 2 (COX-2). Inflammatory stress strongly induces COX-2 in RMICs. To explore the direct effect of mechanical stress on the expression and activity of COX-2, cultured RMICs were subjected to varying amounts of pressure over time using a novel pressure apparatus. COX-2 mRNA and protein were induced following 60 mmHg pressure for 4 and 6 h, respectively. COX-1 mRNA and protein levels were unchanged. PGE(2) production in the RMICs was increased when cells were subjected to 60 mmHg pressure for 6 h and was prevented by a selective COX-2 inhibitor. Pharmacological inhibition indicating that pressure-induced COX-2 expression is dependent on p38 MAPK and biochemical knockdown experiments showed that NF-kappaB might be involved in the COX-2 induction by pressure. Importantly, terminal deoxyneucleotidyl transferase-mediated dUTP nick-end labeling and methylthiazoletetetrazolium assay studies showed that subjecting RMICs to 60 mmHg pressure for 6 h does not affect cell viability, apoptosis, and proliferation. To further examine the regulation of COX-2 in vivo, rats were subjected to unilateral ureteral obstruction (UUO) for 6 and 12 h. COX-2 mRNA and protein level was increased in inner medulla in response to 6- and 12-h UUO. COX-1 mRNA and protein levels were unchanged. These findings suggest that in vitro application of pressure recapitulates the effects on RMICs found after in vivo UUO. This directly implicates pressure as an important regulator of renal COX-2 expression.
Figures




References
-
- Akai Y, Homma T, Burns KD, Yasuda T, Badr KF, Harris RC. Mechanical stretch/relaxation of cultured rat mesangial cells induces protooncogenes and cyclooxygenase. Am J Physiol Cell Physiol 267: C482–C490, 1994 - PubMed
-
- Barrios-Rodiles M, Chadee K. Novel regulation of cyclooxygenase-2 expression and prostaglandin E2 production by IFN-γ in human macrophages. J Immunol 161: 2441–2448, 1998 - PubMed
-
- Broadbelt NV, Stahl PJ, Chen J, Mizrahi M, Lal A, Bozkurt A, Poppas DP, Felsen D. Early upregulation of iNOS mRNA expression and increase in NO metabolites in pressurized renal epithelial cells. Am J Physiol Renal Physiol 293: F1877–F1888, 2007 - PubMed
-
- Campean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse). Am J Physiol Renal Physiol 285: F19–F32, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

