Structural mechanism of C-type inactivation in K(+) channels
- PMID: 20613835
- PMCID: PMC3033749
- DOI: 10.1038/nature09153
Structural mechanism of C-type inactivation in K(+) channels
Abstract
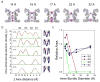
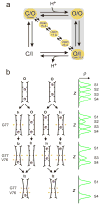
Interconversion between conductive and non-conductive forms of the K(+) channel selectivity filter underlies a variety of gating events, from flicker transitions (at the microsecond timescale) to C-type inactivation (millisecond to second timescale). Here we report the crystal structure of the Streptomyces lividans K(+) channel KcsA in its open-inactivated conformation and investigate the mechanism of C-type inactivation gating at the selectivity filter from channels 'trapped' in a series of partially open conformations. Five conformer classes were identified with openings ranging from 12 A in closed KcsA (Calpha-Calpha distances at Thr 112) to 32 A when fully open. They revealed a remarkable correlation between the degree of gate opening and the conformation and ion occupancy of the selectivity filter. We show that a gradual filter backbone reorientation leads first to a loss of the S2 ion binding site and a subsequent loss of the S3 binding site, presumably abrogating ion conduction. These structures indicate a molecular basis for C-type inactivation in K(+) channels.
Figures
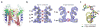

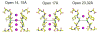
References
-
- Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. - PubMed
-
- Kuo A, et al. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. - PubMed
-
- Jiang Y, et al. The open pore conformation of potassium channels. Nature. 2002;417:523–526. - PubMed
-
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

