Human mucosal associated invariant T cells detect bacterially infected cells
- PMID: 20613858
- PMCID: PMC2893946
- DOI: 10.1371/journal.pbio.1000407
Human mucosal associated invariant T cells detect bacterially infected cells
Abstract
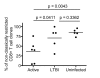
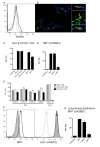
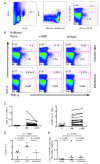
Control of infection with Mycobacterium tuberculosis (Mtb) requires Th1-type immunity, of which CD8+ T cells play a unique role. High frequency Mtb-reactive CD8+ T cells are present in both Mtb-infected and uninfected humans. We show by limiting dilution analysis that nonclassically restricted CD8+ T cells are universally present, but predominate in Mtb-uninfected individuals. Interestingly, these Mtb-reactive cells expressed the Valpha7.2 T-cell receptor (TCR), were restricted by the nonclassical MHC (HLA-Ib) molecule MR1, and were activated in a transporter associated with antigen processing and presentation (TAP) independent manner. These properties are all characteristics of mucosal associated invariant T cells (MAIT), an "innate" T-cell population of previously unknown function. These MAIT cells also detect cells infected with other bacteria. Direct ex vivo analysis demonstrates that Mtb-reactive MAIT cells are decreased in peripheral blood mononuclear cells (PBMCs) from individuals with active tuberculosis, are enriched in human lung, and respond to Mtb-infected MR1-expressing lung epithelial cells. Overall, these findings suggest a generalized role for MAIT cells in the detection of bacterially infected cells, and potentially in the control of bacterial infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- WHO. Geneva: World Health Organization; 2008. Global tuberculosis control - surveillance, planning, financing.
-
- Flynn J. L, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. - PubMed
-
- Lewinsohn D. A, Heinzel A. S, Gardner J. M, Zhu L, Alderson M. R, et al. Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. Am J Respir Crit Care Med. 2003;168:1346–1352. - PubMed
-
- Lewinsohn D. A, Winata E, Swarbrick G. M, Tanner K. E, Cook M. S, et al. Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B. PLoS Pathog. 2007;3:1240–1249. doi: 10.1371/journal.ppat.0030127. - DOI - PMC - PubMed
-
- Lewinsohn D. M, Briden A. L, Reed S. G, Grabstein K. H, Alderson M. R. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J Immunol. 2000;165:925–930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

