Disruption of Dnmt1/PCNA/UHRF1 interactions promotes tumorigenesis from human and mice glial cells
- PMID: 20613874
- PMCID: PMC2894052
- DOI: 10.1371/journal.pone.0011333
Disruption of Dnmt1/PCNA/UHRF1 interactions promotes tumorigenesis from human and mice glial cells
Abstract
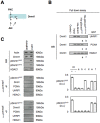
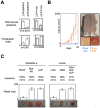
Global DNA hypomethylation is a hallmark of cancer cells, but its molecular mechanisms have not been elucidated. Here, we show that the disruption of Dnmt1/PCNA/UHRF1 interactions promotes a global DNA hypomethylation in human gliomas. We then demonstrate that the Dnmt1 phosphorylations by Akt and/or PKC abrogate the interactions of Dnmt1 with PCNA and UHRF1 in cellular and acellular studies including mass spectrometric analyses and the use of primary cultured patient-derived glioma. By using methylated DNA immunoprecipitation, methylation and CGH arrays, we show that global DNA hypomethylation is associated with genes hypomethylation, hypomethylation of DNA repeat element and chromosomal instability. Our results reveal that the disruption of Dnmt1/PCNA/UHRF1 interactions acts as an oncogenic event and that one of its signatures (i.e. the low level of mMTase activity) is a molecular biomarker associated with a poor prognosis in GBM patients. We identify the genetic and epigenetic alterations which collectively promote the acquisition of tumor/glioma traits by human astrocytes and glial progenitor cells as that promoting high proliferation and apoptosis evasion.
Conflict of interest statement
Figures







References
-
- Feinberg A, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. - PubMed
-
- Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. - PubMed
-
- Hoffmann M, Schulz W. Causes and consequences of DNA hypomethylation in human cancer. Biochem Cell Biol. 2005;83:296–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

