Cyclosporin A treatment of Leishmania donovani reveals stage-specific functions of cyclophilins in parasite proliferation and viability
- PMID: 20614016
- PMCID: PMC2894131
- DOI: 10.1371/journal.pntd.0000729
Cyclosporin A treatment of Leishmania donovani reveals stage-specific functions of cyclophilins in parasite proliferation and viability
Abstract
Background: Cyclosporin A (CsA) has important anti-microbial activity against parasites of the genus Leishmania, suggesting CsA-binding cyclophilins (CyPs) as potential drug targets. However, no information is available on the genetic diversity of this important protein family, and the mechanisms underlying the cytotoxic effects of CsA on intracellular amastigotes are only poorly understood. Here, we performed a first genome-wide analysis of Leishmania CyPs and investigated the effects of CsA on host-free L. donovani amastigotes in order to elucidate the relevance of these parasite proteins for drug development.
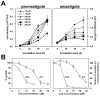
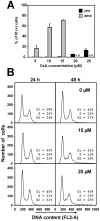
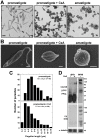
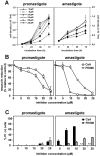
Methodology/principal findings: Multiple sequence alignment and cluster analysis identified 17 Leishmania CyPs with significant sequence differences to human CyPs, but with highly conserved functional residues implicated in PPIase function and CsA binding. CsA treatment of promastigotes resulted in a dose-dependent inhibition of cell growth with an IC50 between 15 and 20 microM as demonstrated by proliferation assay and cell cycle analysis. Scanning electron microscopy revealed striking morphological changes in CsA treated promastigotes reminiscent to developing amastigotes, suggesting a role for parasite CyPs in Leishmania differentiation. In contrast to promastigotes, CsA was highly toxic to amastigotes with an IC50 between 5 and 10 microM, revealing for the first time a direct lethal effect of CsA on the pathogenic mammalian stage linked to parasite thermotolerance, independent from host CyPs. Structural modeling, enrichment of CsA-binding proteins from parasite extracts by FPLC, and PPIase activity assays revealed direct interaction of the inhibitor with LmaCyP40, a bifunctional cyclophilin with potential co-chaperone function.
Conclusions/significance: The evolutionary expansion of the Leishmania CyP protein family and the toxicity of CsA on host-free amastigotes suggest important roles of PPIases in parasite biology and implicate Leishmania CyPs in key processes relevant for parasite proliferation and viability. The requirement of Leishmania CyP functions for intracellular parasite survival and their substantial divergence form host CyPs defines these proteins as prime drug targets.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Lu KP, Liou Y-C, Zhou XZ. Pinning down proline-directed phosphorylation signaling. Trends in Cell Biology. 2002;12:164–172. - PubMed
-
- Dunn CJ, Wagstaff AJ, Perry CM, Plosker GL, Goa KL. Cyclosporin: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral)1 in organ transplantation. Drugs. 2001;61:1957–2016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

