Single dose oral lumiracoxib for postoperative pain in adults
- PMID: 20614451
- PMCID: PMC4164453
- DOI: 10.1002/14651858.CD006865.pub2
Single dose oral lumiracoxib for postoperative pain in adults
Abstract
Background: Lumiracoxib is a selective cyclooxygenase-2 (COX-2) inhibitor. COX-2 inhibitors were developed to avoid COX-1-related gastrointestinal (GI) problems while maintaining the analgesic and anti-inflammatory activity of traditional non-steriodal anti-inflammatory drugs (NSAIDs).
Objectives: To review the analgesic efficacy, duration of analgesia, and adverse effects of a single oral dose of lumiracoxib for moderate to severe postoperative pain in adults.
Search strategy: We searched Cochrane CENTRAL, MEDLINE, and EMBASE to February 2010.
Selection criteria: Single oral dose, randomised, double-blind, placebo-controlled trials of lumiracoxib for relief of established moderate to severe postoperative pain in adults.
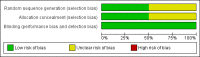
Data collection and analysis: Studies were assessed for methodological quality and the data extracted by two review authors independently. Summed total pain relief over six hours (TOTPAR 6) was used to calculate the number of participants achieving at least 50% pain relief. These derived results were used to calculate, with 95% confidence intervals, the relative benefit compared to placebo, and the number needed to treat (NNT) for one participant to experience at least 50% pain relief over six hours. Numbers of participants using rescue medication, and time to use of rescue medication, were sought as additional measures of efficacy. Information on adverse events and withdrawals was collected.
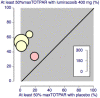
Main results: In this updated review four studies met the inclusion criteria. In total 366 participants were treated with lumiracoxib 400 mg, 51 with lumiracoxib 100 mg, and 212 with placebo. Active comparators were naproxen 500 mg, rofecoxib 50 mg, celecoxib 200 mg, celecoxib 400 mg, and ibuprofen 400 mg. With lumiracoxib 400 mg 50% of participants had at least 50% pain relief over six hours, compared with 8% given placebo; RB 6.9 (95% CI 4.1 to 12), NNT 2.4 (2.1 to 2.8).Median time to onset of analgesia was shorter for lumiracoxib 400 mg (0.6 to 1.5 hours) than placebo (>12 hours). Fewer participants needed rescue medication with lumiracoxib (64%) than with placebo (91%) over 12 to 24 hours; NNT to prevent remedication 3.7 (2.9 to 5.0). The weighted median time to use of rescue medication was 9.4 hours for lumiracoxib 400 mg and 1.7 hours for placebo.Adverse events were generally mild to moderate in severity, with one serious event reported in a placebo patient.
Authors' conclusions: Lumiracoxib 400 mg given as a single oral dose is an effective analgesic for acute postoperative pain, and has a relatively long duration of action. Adverse events with lumiracoxib did not differ from placebo.
Conflict of interest statement
SD and RAM have received research support from charities, government and industry sources at various times, but no such support was received for this work. RAM and HJM have consulted for various pharmaceutical companies. RAM has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. YMR is employed by the PaPaS Review Group.
Figures




Update of
-
Single dose oral lumiracoxib for postoperative pain.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD006865. doi: 10.1002/14651858.CD006865. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2010 Jul 07;(7):CD006865. doi: 10.1002/14651858.CD006865.pub2. PMID: 17943921 Free PMC article. Updated.
References
References to studies included in this review
Chan 2005 {published data only}
-
- Chan VWS, Clark A J, Davis JC, Wolf RS, Kellstein D, Jayawardene S. The post‐operative analgesic efficacy and tolerability of lumiracoxib compared with placebo and naproxen after total knee or hip arthroplasty. Acta Anaesthesiologica Scandinavica 2005;Nov 49(10):1491‐500. - PubMed
Fricke 2008 {published data only}
Kellstein 2004 {published data only}
-
- Kellstein D, Ott D, Jayawardene S, Fricke J. Analgesic efficacy of a single dose of lumiracoxib compared with rofecoxib, celecoxib and placebo in the treatment of post‐operative dental pain. International Journal of Clinical Practice 2004;58(3):244‐50. - PubMed
Zelenakas 2004 {published data only}
-
- Zelenakas K, Fricke JR Jr, Jayawardene S, Kellstein D. Analgesic efficacy of single oral doses of lumiracoxib and ibuprofen in patients with postoperative dental pain. International Journal of Clinical Practice 2004;58(3):251‐6. - PubMed
References to studies excluded from this review
Bitner 2004 {published data only}
-
- Bitner M, Katterhorn J, Hatfield C, Gao J, Kellstein D. Efficacy and tolerability of lumiracoxib in the treatment of primary dysmenorrhoea. International Journal Clinical Practice 2004;58(4):340‐5. - PubMed
Schnitzer 2004 {published data only}
-
- Schnitzer TJ, Beier J, Geusens P, Hasler P, Patel SK, Senftleber I, et al. Efficacy and safety of four doses of lumiracoxib versus diclofenac in patients with knee or hip primary osteoarthritis: A phase II, four week, multicenter randomised, double blind, placebo‐controlled trial. Arthritis and Rheumatism 2004;51(4):549‐57. - PubMed
Additional references
Bannwarth 2005
-
- Bannworth B, Berenbaum F. Clinical pharmacology of lumiracoxib, a second generation cyclooxygenase 2 selective inhibitor. Expert Opinion on Investigational Drugs 2005;14(4):521‐33. - PubMed
Barden 2004
-
- Barden J, Edwards JE, McQuay HJ, Moore RA. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain 2004;107:86‐90. - PubMed
Barden 2006
Clarke 2009
Collins 2001
-
- Collins SL, Edwards J, Moore RA, Smith LA, McQuay HJ. Seeking a simple measure of analgesia for mega‐trials: is a single global assessment good enough?. Pain 2001;91((1‐2)):189‐94. - PubMed
Cook 1995
Cooper 1991
-
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM editor(s). The design of analgesic clinical trials. Advances in Pain Research and Therapy. Vol. 18, New York: Raven Press, 1991:117‐24.
Day 1988
-
- Day RO, McLachlan AJ, Graham GG, Williams KM. Pharmokinetics of non‐steroidal anti‐inflammatory drugs in synovial fluid. Baillieres Clinical Rheumatology 1988;2:363‐93. - PubMed
Derry 2008
Derry C 2009a
Derry C 2009b
Derry P 2009
Fitzgerald 2001
-
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. New England Journal of Medicine 2001;345(6):433‐42. - PubMed
Forrest 2002
-
- Forrest JB, Camu F, Greer IA, Kehlet H, Abdalla M, Bonnet F. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery. British Journal of Anaesthesia 2002;88(2):227‐33. - PubMed
Garner 2002
Grahame‐Smith 2002
-
- Grahame‐Smith DG, Aronson JK. Oxford textbook of clinical pharmacology and drug therapy. 3rd Edition. Oxford: Oxford University Press, 2002.
Hawkey 1999
-
- Hawkey CJ. Cox‐2 inhibitors. Lancet 1999;353(9149):307‐14. - PubMed
Hawkey 2001
-
- Hawkey CJ. Gastrointestinal safety of COX‐2 specific inhibitors. Gastroenterology Clinics of North America 2001;30(4):921‐36. - PubMed
Hawkey 2006
-
- Hawkey CJ. NSAIDs, coxibs, and the intestine. Journal of Cardiovascular Pharmacology 2006;47(Suppl 1):S72‐5. - PubMed
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Kearney 2006
L'Abbe 1987
-
- L'Abbe KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
Lloyd 2009
McQuay 2005
Moher 1999
-
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of meta‐analyses of randomised controlled trials: the QUOROM statement. Lancet 1999;354:1896‐900. - PubMed
Moore 1996
-
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2‐3):229‐37. - PubMed
Moore 1997a
-
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Verification from independent data. Pain 1997;69(1‐2):127‐30. - PubMed
Moore 1997b
-
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Use of pain intensity and visual analogue scales. Pain 1997;69(3):311‐5. - PubMed
Moore 1998
-
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. - PubMed
Moore 2003
-
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003. [ISBN: 0‐19‐263247‐7]
Moore 2005a
-
- Moore RA, Edwards JE, McQuay HJM. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116:322‐31. - PubMed
Moore 2005b
-
- Moore RA, Derry S, Makinson GT, McQuay HJ. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta‐analysis of information from company clinical trial reports. Arthritis Research and Therapeutics 2005;7(3):R644‐65. - PMC - PubMed
Moore 2006
-
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2]
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Morris 1995
-
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratio and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with confidence ‐ confidence intervals and statistical guidelines. London: BMJ, 1995:50‐63.
Mysler 2004
-
- Mysler E. Lumiracoxib (Prexige): a new selective COX‐2 inhibitor. International Journal of Clinical Practice 2004;58(6):606‐11. - PubMed
Patrono 2009
Schnitzer 2004a
-
- Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004;364(9435):665‐74. - PubMed
Toms 2008
Toms 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

