Generation of mice with a conditional null allele for Wntless
- PMID: 20614471
- PMCID: PMC3689319
- DOI: 10.1002/dvg.20651
Generation of mice with a conditional null allele for Wntless
Abstract
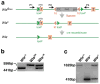
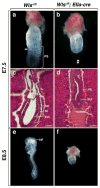
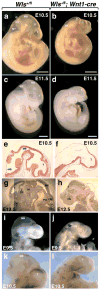
The Wnt-signaling pathway is necessary in a variety of developmental processes and has been implicated in numerous pathologies. Wntless (Wls) binds to Wnt proteins and facilitates Wnt sorting and secretion. Conventional deletion of Wls results in early fetal lethality due to defects in body axis establishment. To gain insight into the function of Wls in later stages of development, we have generated a conditional null allele. Homozygous germline deletion of Wls confirmed prenatal lethality and failure of embryonic axis formation. Deletion of Wls using Wnt1-cre phenocopied Wnt1 null abnormalities in the midbrain and hindbrain. In addition, conditional deletion of Wls in pancreatic precursor cells resulted in pancreatic hypoplasia similar to that previously observed after conditional β-catenin deletion. This Wls conditional null allele will be valuable in detecting novel Wnt functions in development and disease.
© 2010 Wiley-Liss, Inc.
Figures

References
-
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. - PubMed
-
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. - PubMed
-
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling Wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. - PubMed
-
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. - PubMed
-
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

