Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A
- PMID: 20614936
- PMCID: PMC2916045
- DOI: 10.1021/ja102758v
Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A
Abstract
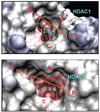
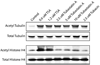
Structure-based drug design combined with homology modeling techniques were used to develop potent inhibitors of HDAC6 that display superior selectivity for the HDAC6 isozyme compared to other inhibitors. These inhibitors can be assembled in a few synthetic steps, and thus are readily scaled up for in vivo studies. An optimized compound from this series, designated Tubastatin A, was tested in primary cortical neuron cultures in which it was found to induce elevated levels of acetylated alpha-tubulin, but not histone, consistent with its HDAC6 selectivity. Tubastatin A also conferred dose-dependent protection in primary cortical neuron cultures against glutathione depletion-induced oxidative stress. Importantly, when given alone at all concentrations tested, this hydroxamate-containing HDAC6-selective compound displayed no neuronal toxicity, thus, forecasting the potential application of this agent and its analogues to neurodegenerative conditions.
Figures
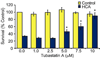
References
-
- Xu WS, Parmigiani RB, Marks PA. Oncogene. 2007;26:5541–5552. - PubMed
-
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Science. 2009;325:834–840. - PubMed
-
- Kelly WK, Marks PA. Nat. Clin. Pract. Oncol. 2005;2:150–157. - PubMed
-
- Dokmanovic M, Clarke C, Marks PA. Mol. Cancer Res. 2007;5:981–989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

