Biofoams and natural protein surfactants
- PMID: 20615601
- PMCID: PMC2954283
- DOI: 10.1016/j.bpc.2010.06.006
Biofoams and natural protein surfactants
Abstract
Naturally occurring foam constituent and surfactant proteins with intriguing structures and functions are now being identified from a variety of biological sources. The ranaspumins from tropical frog foam nests comprise a range of proteins with a mixture of surfactant, carbohydrate binding and antimicrobial activities that together provide a stable, biocompatible, protective foam environment for developing eggs and embryos. Ranasmurfin, a blue protein from a different species of frog, displays a novel structure with a unique chromophoric crosslink. Latherin, primarily from horse sweat, but with similarities to salivary, oral and upper respiratory tract proteins, illustrates several potential roles for surfactant proteins in mammalian systems. These proteins, together with the previously discovered hydrophobins of fungi, throw new light on biomolecular processes at air-water and other interfaces. This review provides a perspective on these recent findings, focussing on structure and biophysical properties.
2010 Elsevier B.V. All rights reserved.
Figures

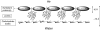
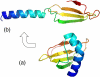
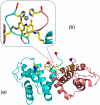

References
-
- Clarkson J.R., Cui Z.F., Darton R.C., Clarkson J.R. Protein denaturation in foam — I. Mechanism study. J. Colloid Interface Sci. 1999;215:323–332. - PubMed
-
- Clarkson J.R., Cui Z.F., Darton R.C. Protein denaturation in foam — II. Surface activity and conformational change. J. Colloid Interface Sci. 1999;215:333–338. - PubMed
-
- Lu J.R., Zhao X.B., Yaseen M. Biomimetic amphiphiles: biosurfactants. Curr. Opin. Colloid Interface Sci. 2007;12:60–67.
-
- Ron E.Z., Rosenberg E. Natural roles of biosurfactants. Environ. Microbiol. 2001;3:229–236. - PubMed
-
- Holmberg K. Natural surfactants. Curr. Opin. Colloid Interface Sci. 2001;6:148–159.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

