Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer
- PMID: 20615629
- PMCID: PMC2952351
- DOI: 10.1016/j.ijrobp.2010.03.012
Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer
Abstract
Purpose: To minimize toxicity while maintaining tumor coverage with stereotactic body radiation therapy (SBRT) for centrally or superiorly located stage I non-small-cell lung cancer (NSCLC), we investigated passive-scattering proton therapy (PSPT) and intensity-modulated proton therapy (IMPT).
Methods and materials: Fifteen patients with centrally or superiorly located (within 2 cm of critical structures) stage I NSCLC were treated clinically with three-dimensional photon SBRT (50 Gy in 4 fractions). The photon SBRT plan was compared with the PSPT and IMPT plans. The maximum tolerated dose (MTD) was defined as the dose that exceeded the dose--volume constraints in the critical structures.
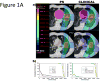
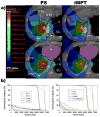
Results: Only 6 photon plans satisfied the >95% planning target volume (PTV) coverage and MTD constraints, compared to 12 PSPT plans (p = 0.009) and 14 IMPT plans (p = 0.001). Compared with the photon SBRT plans, the PSPT and IMPT plans significantly reduced the mean total lung dose from 5.4 Gy to 3.5 Gy (p < 0.001) and 2.8 Gy (p < 0.001) and reduced the total lung volume receiving 5 Gy, 10 Gy, and 20 Gy (p < 0.001). When the PTV was within 2 cm of the critical structures, the PSPT and IMPT plans significantly reduced the mean maximal dose to the aorta, brachial plexus, heart, pulmonary vessels, and spinal cord.
Conclusions: For centrally or superiorly located stage I NSCLC, proton therapy, particularly IMPT, delivered ablative doses to the target volume and significantly reduced doses to the surrounding normal tissues compared with photon SBRT.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Notification: Actual or potential conflicts of interest do not exist.
Figures




Comment in
-
Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer: in regards to Register et al. Int J Radiat Oncol Biol Phys 2011;80:1015-1022.Int J Radiat Oncol Biol Phys. 2012 Jan 1;82(1):492; author reply 492. doi: 10.1016/j.ijrobp.2011.08.042. Int J Radiat Oncol Biol Phys. 2012. PMID: 22182718 No abstract available.
References
-
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–100. - PubMed
-
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. - PubMed
-
- Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1087–1096. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

