Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves
- PMID: 20615870
- PMCID: PMC2934668
- DOI: 10.1074/jbc.M110.125336
Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves
Abstract
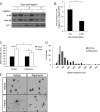
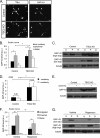
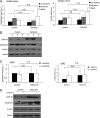
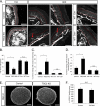
Unlike neurons in the central nervous system (CNS), injured neurons in the peripheral nervous system (PNS) can regenerate their axons and reinnervate their targets. However, functional recovery in the PNS often remains suboptimal, especially in cases of severe damage. The lack of regenerative ability of CNS neurons has been linked to down-regulation of the mTOR (mammalian target of rapamycin) pathway. We report here that PNS dorsal root ganglial neurons (DRGs) activate mTOR following damage and that this activity enhances axonal growth capacity. Furthermore, genetic up-regulation of mTOR activity by deletion of tuberous sclerosis complex 2 (TSC2) in DRGs is sufficient to enhance axonal growth capacity in vitro and in vivo. We further show that mTOR activity is linked to the expression of GAP-43, a crucial component of axonal outgrowth. However, although TSC2 deletion in DRGs facilitates axonal regrowth, it leads to defects in target innervation. Thus, whereas manipulation of mTOR activity could provide new strategies to stimulate nerve regeneration in the PNS, fine control of mTOR activity is required for proper target innervation.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

