Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury
- PMID: 20615974
- PMCID: PMC2906548
- DOI: 10.1073/pnas.0910106107
Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury
Abstract
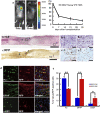
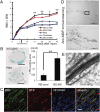
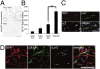
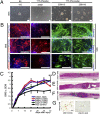
Various types of induced pluripotent stem (iPS) cells have been established by different methods, and each type exhibits different biological properties. Before iPS cell-based clinical applications can be initiated, detailed evaluations of the cells, including their differentiation potentials and tumorigenic activities in different contexts, should be investigated to establish their safety and effectiveness for cell transplantation therapies. Here we show the directed neural differentiation of murine iPS cells and examine their therapeutic potential in a mouse spinal cord injury (SCI) model. "Safe" iPS-derived neurospheres, which had been pre-evaluated as nontumorigenic by their transplantation into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse brain, produced electrophysiologically functional neurons, astrocytes, and oligodendrocytes in vitro. Furthermore, when the safe iPS-derived neurospheres were transplanted into the spinal cord 9 d after contusive injury, they differentiated into all three neural lineages without forming teratomas or other tumors. They also participated in remyelination and induced the axonal regrowth of host 5HT(+) serotonergic fibers, promoting locomotor function recovery. However, the transplantation of iPS-derived neurospheres pre-evaluated as "unsafe" showed robust teratoma formation and sudden locomotor functional loss after functional recovery in the SCI model. These findings suggest that pre-evaluated safe iPS clone-derived neural stem/progenitor cells may be a promising cell source for transplantation therapy for SCI.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Björklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. - PubMed
-
- Okano H. Stem cell biology of the central nervous system. J Neurosci Res. 2002;69:698–707. - PubMed
-
- Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders—how to make it work. Nat Med. 2004;10(Suppl):S42–S50. - PubMed
-
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. - PubMed
-
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

