Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge
- PMID: 20615991
- PMCID: PMC2922242
- DOI: 10.1073/pnas.1007465107
Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge
Abstract
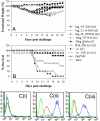
Influenza HA is the primary target of neutralizing antibodies during infection, and its sequence undergoes genetic drift and shift in response to immune pressure. The receptor binding HA1 subunit of HA shows much higher sequence variability relative to the metastable, fusion-active HA2 subunit, presumably because neutralizing antibodies are primarily targeted against the former in natural infection. We have designed an HA2-based immunogen using a protein minimization approach that incorporates designed mutations to destabilize the low pH conformation of HA2. The resulting construct (HA6) was expressed in Escherichia coli and refolded from inclusion bodies. Biophysical studies and mutational analysis of the protein indicate that it is folded into the desired neutral pH conformation competent to bind the broadly neutralizing HA2 directed monoclonal 12D1, not the low pH conformation observed in previous studies. HA6 was highly immunogenic in mice and the mice were protected against lethal challenge by the homologous A/HK/68 mouse-adapted virus. An HA6-like construct from another H3 strain (A/Phil/2/82) also protected mice against A/HK/68 challenge. Regions included in HA6 are highly conserved within a subtype and are fairly well conserved within a clade. Targeting the highly conserved HA2 subunit with a bacterially produced immunogen is a vaccine strategy that may aid in pandemic preparedness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
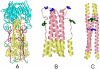
Comment in
-
Stalking influenza.Proc Natl Acad Sci U S A. 2010 Aug 3;107(31):13563-4. doi: 10.1073/pnas.1008672107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660754 Free PMC article. No abstract available.
References
-
- Fan J, et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004;22:2993–3003. - PubMed
-
- Horimoto T, Kawaoka Y. Influenza: Lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. - PubMed
-
- Cox MM. Cell-based protein vaccines for influenza. Curr Opin Mol Ther. 2005;7:24–9. - PubMed
-
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. - PubMed
-
- Chen J, et al. Structure of the hemagglutinin precursor cleavage site, a determinant of influenza pathogenicity and the origin of the labile conformation. Cell. 1998;95:409–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

