A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome
- PMID: 20615998
- PMCID: PMC3063586
- DOI: 10.1073/pnas.1000066107
A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome
Abstract
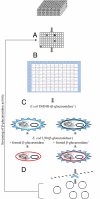
In the human gastrointestinal tract, bacterial β-D-glucuronidases (BG; E.C. 3.2.1.31) are involved both in xenobiotic metabolism and in some of the beneficial effects of dietary compounds. Despite their biological significance, investigations are hampered by the fact that only a few BGs have so far been studied. A functional metagenomic approach was therefore performed on intestinal metagenomic libraries using chromogenic glucuronides as probes. Using this strategy, 19 positive metagenomic clones were identified but only one exhibited strong β-D-glucuronidase activity when subcloned into an expression vector. The cloned gene encoded a β-D-glucuronidase (called H11G11-BG) that had distant amino acid sequence homologies and an additional C terminus domain compared with known β-D-glucuronidases. Fifteen homologs were identified in public bacterial genome databases (38-57% identity with H11G11-BG) in the Firmicutes phylum. The genomes identified derived from strains from Ruminococcaceae, Lachnospiraceae, and Clostridiaceae. The genetic context diversity, with closely related symporters and gene duplication, argued for functional diversity and contribution to adaptive mechanisms. In contrast to the previously known β-D-glucuronidases, this previously undescribed type was present in the published microbiome of each healthy adult/child investigated (n = 11) and was specific to the human gut ecosystem. In conclusion, our functional metagenomic approach revealed a class of BGs that may be part of a functional core specifically evolved to adapt to the human gut environment with major health implications. We propose consensus motifs for this unique Firmicutes β-D-glucuronidase subfamily and for the glycosyl hydrolase family 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rod TO, Midtvedt T. Origin of intestinal beta-glucuronidase in germfree, monocontaminated and conventional rats. Acta Pathol Microbiol Scand [B] 1977;85:271–276. - PubMed
-
- Knasmuller S, et al. Impact of bacteria in dairy products and of the intestinal microflora on the genotoxic and carcinogenic effects of heterocyclic aromatic amines. Mutat Res. 2001;480–481:129–138. - PubMed
-
- Humblot C, et al. Beta-glucuronidase in human intestinal microbiota is necessary for the colonic genotoxicity of the food-borne carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline in rats. Carcinogenesis. 2007;28:2419–2425. - PubMed
-
- de Herder WW, Hazenberg MP, Pennock-Schroder AM, Hennemann G, Visser TJ. Rapid and bacteria-dependent in vitro hydrolysis of iodothyronine-conjugates by intestinal contents of humans and rats. Med Biol. 1986;64:31–35. - PubMed
-
- Graef V, Furuya E, Nishikaze O. Hydrolysis of steroid glucuronides with beta-glucuronidase preparations from bovine liver, Helix pomatia, and E. coli. Clin Chem. 1977;23:532–535. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

