Chemical genetic strategy identifies histone deacetylase 1 (HDAC1) and HDAC2 as therapeutic targets in sickle cell disease
- PMID: 20616024
- PMCID: PMC2906581
- DOI: 10.1073/pnas.1006774107
Chemical genetic strategy identifies histone deacetylase 1 (HDAC1) and HDAC2 as therapeutic targets in sickle cell disease
Abstract
The worldwide burden of sickle cell disease is enormous, with over 200,000 infants born with the disease each year in Africa alone. Induction of fetal hemoglobin is a validated strategy to improve symptoms and complications of this disease. The development of targeted therapies has been limited by the absence of discrete druggable targets. We developed a unique bead-based strategy for the identification of inducers of fetal hemoglobin transcripts in primary human erythroid cells. A small-molecule screen of bioactive compounds identified remarkable class-associated activity among histone deacetylase (HDAC) inhibitors. Using a chemical genetic strategy combining focused libraries of biased chemical probes and reverse genetics by RNA interference, we have identified HDAC1 and HDAC2 as molecular targets mediating fetal hemoglobin induction. Our findings suggest the potential of isoform-selective inhibitors of HDAC1 and HDAC2 for the treatment of sickle cell disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
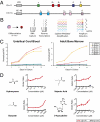
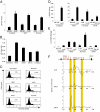
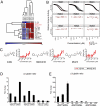


References
-
- Weatherall D, Akinyanju O, Fucharoen S, Olivier N, Musgrove P. In: Disease Control Priorities in Developing Countries. Jamieson D, et al., editors. Oxford, Washington: Oxford University Press; 2006. pp. 663–680.
-
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–769. - PubMed
-
- Letvin NL, Linch DC, Beardsley GP, McIntyre KW, Nathan DG. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984;310:869–873. - PubMed
-
- Charache S, et al. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332:1317–1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

