Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses
- PMID: 20616031
- PMCID: PMC2906563
- DOI: 10.1073/pnas.1007270107
Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses
Abstract
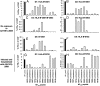
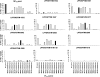
Preexisting T-cell immunity directed at conserved viral regions promotes enhanced recovery from influenza virus infections, with there being some evidence of cross-protection directed at variable peptides. Strikingly, many of the immunogenic peptides derived from the current pandemic A(H1N1)-2009 influenza virus are representative of the catastrophic 1918 "Spanish flu" rather than more recent "seasonal" strains. We present immunological and structural analyses of cross-reactive CD8(+) T-cell-mediated immunity directed at a variable (although highly cross-reactive) immunodominant NP(418-426) peptide that binds to a large B7 family (HLA-B*3501/03/0702) found throughout human populations. Memory CD8(+) T-cell specificity was probed for 12 different NP(418) mutants that emerged over the 9 decades between the 1918 and 2009 pandemics. Although there is evidence of substantial cross-reactivity among seasonal NP(418) mutants, current memory T-cell profiles show no preexisting immunity to the 2009-NP(418) variant or the 1918-NP(418) variant. Natural infection with the A(H1N1)-2009 virus, however, elicits CD8(+) T cells specific for the 2009-NP(418) and 1918-NP(418) epitopes. This analysis points to the potential importance of cross-reactive T-cell populations that cover the possible spectrum of T-cell variants and suggests that the identification of key residues/motifs that elicit cross-reactive T-cell sets could facilitate the evolution of immunization protocols that provide a measure of protection against unpredicted pandemic influenza viruses. Thus, it is worth exploring the potential of vaccines that incorporate peptide variants with a proven potential for broader immunogenicity, especially to those that are not recognized by the current memory T-cell pool generated by exposure to influenza variants that cause successive seasonal epidemics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

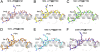
References
-
- Cohen J, Enserink M. Swine flu. After delays, WHO agrees: The 2009 pandemic has begun. Science. 2009;324:1496–1497. - PubMed
-
- Fisman DN, et al. Older age and a reduced likelihood of 2009 H1N1 virus infection. N Engl J Med. 2009;361:2000–2001. - PubMed
-
- Epstein SL. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: An experiment of nature. J Infect Dis. 2006;193:49–53. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

