Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation
- PMID: 20616036
- PMCID: PMC2906549
- DOI: 10.1073/pnas.1006542107
Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation
Abstract
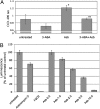
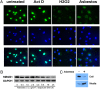
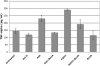
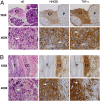
Asbestos carcinogenesis has been linked to the release of cytokines and mutagenic reactive oxygen species (ROS) from inflammatory cells. Asbestos is cytotoxic to human mesothelial cells (HM), which appears counterintuitive for a carcinogen. We show that asbestos-induced HM cell death is a regulated form of necrosis that links to carcinogenesis. Asbestos-exposed HM activate poly(ADP-ribose) polymerase, secrete H(2)O(2), deplete ATP, and translocate high-mobility group box 1 protein (HMGB1) from the nucleus to the cytoplasm, and into the extracellular space. The release of HMGB1 induces macrophages to secrete TNF-alpha, which protects HM from asbestos-induced cell death and triggers a chronic inflammatory response; both favor HM transformation. In both mice and hamsters injected with asbestos, HMGB1 was specifically detected in the nuclei, cytoplasm, and extracellular space of mesothelial and inflammatory cells around asbestos deposits. TNF-alpha was coexpressed in the same areas. HMGB1 levels in asbestos-exposed individuals were significantly higher than in nonexposed controls (P < 0.0001). Our findings identify the release of HMGB1 as a critical initial step in the pathogenesis of asbestos-related disease, and provide mechanistic links between asbestos-induced cell death, chronic inflammation, and carcinogenesis. Chemopreventive approaches aimed at inhibiting the chronic inflammatory response, and especially blocking HMGB1, may decrease the risk of malignant mesothelioma among asbestos-exposed cohorts.
Conflict of interest statement
Conflict of interest statement: M.E.B. is founder and part owner of HMGBiotech, a company that provides goods and services related to HMGB proteins.
Figures


References
-
- Carbone M, Bedrossian CW. The pathogenesis of mesothelioma. Semin Diagn Pathol. 2006;23:56–60. - PubMed
-
- Carbone M, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7:147–154. - PubMed
-
- Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433–439. - PubMed
-
- Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

