Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells
- PMID: 20616080
- PMCID: PMC2922145
- DOI: 10.1073/pnas.1007884107
Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells
Abstract
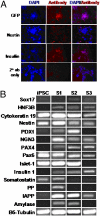
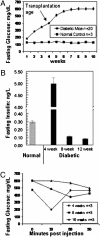
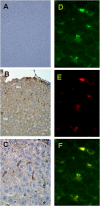
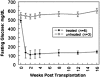
Diabetes mellitus is characterized by either the inability to produce insulin (type 1 diabetes) or as insensitivity to insulin secreted by the body (type 2 diabetes). In either case, the body is unable to move blood glucose efficiently across cell membranes to be used. This leads to a variety of local and systemic detrimental effects. Current treatments for diabetes focus on exogenous insulin administration and dietary control. Here, we describe a potential cure for diabetes using a cellular therapy to ameliorate symptoms associated with both reduced insulin secretion and insulin sensitivity. Using induced pluripotent stem (iPS) cells, we were able to derive beta-like cells similar to the endogenous insulin-secreting cells in mice. These beta-like cells secreted insulin in response to glucose and corrected a hyperglycemic phenotype in two mouse models of type 1 and 2 diabetes via an iPS cell transplant. Long-term correction of hyperglycemia was achieved, as determined by blood glucose and hemoglobin A1c levels. These data provide an initial proof of principle for potential clinical applications of reprogrammed somatic cells in the treatment of diabetes type 1 or 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jones PM, Courtney ML, Burns CJ, Persaud SJ. Cell-based treatments for diabetes. Drug Discov Today. 2008;13:888–893. - PubMed
-
- Urbán VS, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–253. - PubMed
-
- Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–2844. - PubMed
-
- Sun B, Roh KH, Lee SR, Lee YS, Kang KS. Induction of human umbilical cord blood-derived stem cells with embryonic stem cell phenotypes into insulin producing islet-like structure. Biochem Biophys Res Commun. 2007;354:919–923. - PubMed
-
- Lumelsky N, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

