HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis
- PMID: 20616092
- PMCID: PMC2901470
- DOI: 10.1073/pnas.1006061107
HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis
Abstract
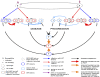
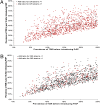
The administration of antiretrovirals before HIV exposure to prevent infection (i.e., preexposure prophylaxis; PrEP) is under evaluation in clinical trials. Because PrEP is based on antiretrovirals, there is considerable concern that it could substantially increase transmitted resistance, particularly in resource-rich countries. Here we use a mathematical model to predict the effect of PrEP interventions on the HIV epidemic in the men-who-have-sex-with-men community in San Francisco. The model is calibrated using Monte Carlo filtering and analyzed by constructing nonlinear response hypersurfaces. We predict PrEP interventions could substantially reduce transmission but significantly increase the proportion of new infections caused by resistant strains. Two mechanisms can cause this increase. If risk compensation occurs, the proportion increases due to increasing transmission of resistant strains and decreasing transmission of wild-type strains. If risk behavior remains stable, the increase occurs because of reduced transmission of resistant strains coupled with an even greater reduction in transmission of wild-type strains. We define this as the paradox of PrEP (i.e., resistance appears to be increasing, but is actually decreasing). We determine this paradox is likely to occur if the efficacy of PrEP regimens against wild-type strains is greater than 30% and the relative efficacy against resistant strains is greater than 0.2 but less than the efficacy against wild-type. Our modeling shows, if risk behavior increases, that it is a valid concern that PrEP could significantly increase transmitted resistance. However, if risk behavior remains stable, we find the concern is unfounded and PrEP interventions are likely to decrease transmitted resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Paxton LA, Hope T, Jaffe HW. Pre-exposure prophylaxis for HIV infection: What if it works? Lancet. 2007;370:89–93. - PubMed
-
- AVAC 2010. PrEP clinical trials. Available at http://www.avac.org/ht/d/sp/i/3507/pid/3507. Accessed April 30, 2010.
-
- Abdool Karim SS, Baxter C. Antiretroviral prophylaxis for the prevention of HIV infection: Future implementation challenges. HIV Ther. 2009;3:3–6.
-
- Booth CL, Geretti AM. Prevalence and determinants of transmitted antiretroviral drug resistance in HIV-1 infection. J Antimicrob Chemother. 2007;59:1047–1056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

