Apical sodium-dependent bile acid transporter upregulation is associated with necrotizing enterocolitis
- PMID: 20616306
- PMCID: PMC2950692
- DOI: 10.1152/ajpgi.00242.2010
Apical sodium-dependent bile acid transporter upregulation is associated with necrotizing enterocolitis
Abstract
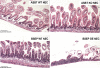
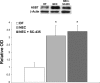
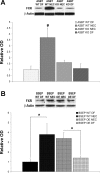
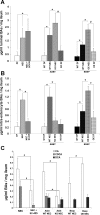
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency of premature infants. Previously, we showed that luminal bile acids (BAs) are increased and correlated with disease development and that the apical sodium-dependent BA transporter (ASBT), which transports BAs from the ileal lumen into enterocytes, is upregulated in rats with NEC. We hypothesized that intraenterocyte, rather than luminal, BAs are associated with NEC and that upregulation of ASBT may be a mechanism by which this occurs. Neonatal rats with or without the ASBT inhibitor SC-435, mice in which ASBT was knocked out, and mice that overproduce BAs were subjected to the NEC protocol. Disease development, ASBT, and the farnesoid X receptor protein, along with luminal and intraenterocyte BA levels, were assessed. In addition, ileal sections from premature infants with and without NEC were examined for ASBT via immunohistology and real-time PCR. When BAs were not transported into enterocytes (rats given SC-435 and ASBT knockout mice), severity and incidence of NEC were reduced. In contrast, in mice that overproduce BAs, ASBT was elevated, intraenterocyte BAs were increased, and disease development was increased. ASBT staining was more intense on the apical membrane of ileal enterocytes from premature infants with NEC than premature infants with non-NEC diagnoses. In addition, ASBT mRNA levels were significantly higher in infants with NEC. These data show that accumulation of intraenterocyte BAs contributes to disease development, elevated ASBT increases disease severity in experimental models of NEC, and ASBT is elevated in human NEC. These data confirm that BAs and upregulation of ASBT play a crucial role in NEC pathogenesis and suggest that inhibition of ASBT could be utilized as a therapeutic modality against this disease.
Figures





Similar articles
-
Active transport of bile acids decreases mucin 2 in neonatal ileum: implications for development of necrotizing enterocolitis.PLoS One. 2011;6(12):e27191. doi: 10.1371/journal.pone.0027191. Epub 2011 Dec 5. PLoS One. 2011. PMID: 22162748 Free PMC article.
-
Clostridium scindens exacerbates experimental necrotizing enterocolitis via upregulation of the apical sodium-dependent bile acid transporter.Am J Physiol Gastrointest Liver Physiol. 2024 Jan 1;326(1):G25-G37. doi: 10.1152/ajpgi.00102.2023. Epub 2023 Nov 7. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 37933481 Free PMC article.
-
Bile acids induce ileal damage during experimental necrotizing enterocolitis.Gastroenterology. 2006 Feb;130(2):359-72. doi: 10.1053/j.gastro.2005.10.023. Gastroenterology. 2006. PMID: 16472592 Free PMC article.
-
Does abnormal bile acid metabolism contribute to NEC?Semin Perinatol. 2008 Apr;32(2):114-21. doi: 10.1053/j.semperi.2008.01.005. Semin Perinatol. 2008. PMID: 18346535 Free PMC article. Review.
-
Apical sodium-dependent bile acid transporter, drug target for bile acid related diseases and delivery target for prodrugs: Current and future challenges.Pharmacol Ther. 2020 Aug;212:107539. doi: 10.1016/j.pharmthera.2020.107539. Epub 2020 Mar 20. Pharmacol Ther. 2020. PMID: 32201314 Review.
Cited by
-
Intestinal Absorption of Bile Acids in Health and Disease.Compr Physiol. 2019 Dec 18;10(1):21-56. doi: 10.1002/cphy.c190007. Compr Physiol. 2019. PMID: 31853951 Free PMC article. Review.
-
Prophylactic Intra-Uterine β-Cyclodextrin Administration during Intra-Uterine Ureaplasma parvum Infection Partly Prevents Liver Inflammation without Interfering with the Enterohepatic Circulation of the Fetal Sheep.Nutrients. 2020 May 5;12(5):1312. doi: 10.3390/nu12051312. Nutrients. 2020. PMID: 32380648 Free PMC article.
-
Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling.Am J Physiol Gastrointest Liver Physiol. 2016 Jan 15;310(2):G81-92. doi: 10.1152/ajpgi.00065.2015. Epub 2015 Nov 25. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26608185 Free PMC article.
-
Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis.Pathophysiology. 2014 Feb;21(1):81-93. doi: 10.1016/j.pathophys.2013.11.007. Epub 2013 Dec 22. Pathophysiology. 2014. PMID: 24365655 Free PMC article.
-
Metabolic immaturity and breastmilk bile acid metabolites are central determinants of heightened newborn vulnerability to norovirus diarrhea.Cell Host Microbe. 2024 Sep 11;32(9):1488-1501.e5. doi: 10.1016/j.chom.2024.08.003. Epub 2024 Aug 29. Cell Host Microbe. 2024. PMID: 39214086
References
-
- Armand M, Hamosh M, Mehta NR, Angelus PA, Philpott JR, Henderson TR, Dwyer NK, Lairon D, Hamosh P. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res 40: 429–437, 1996 - PubMed
-
- Arrese M, Trauner M, Sacchiero RJ, Crossman MW, Shneider BL. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology 28: 1081–1087, 1998 - PubMed
-
- Balistreri WF, Heubi JE, Suchy FJ. Immaturity of the enterohepatic circulation in early life: factors predisposing to “physiologic” maldigestion and cholestasis. J Pediatr Gastroenterol Nutr 2: 346–354, 1983 - PubMed
-
- Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr 117: S6–S13, 1990 - PubMed
-
- Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, Schullinger JN. An experimental study of acute neonatal enterocolitis—the importance of breast milk. J Pediatr Surg 9: 587–595, 1974 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

