Mitochondria control functional CaV1.2 expression in smooth muscle cells of cerebral arteries
- PMID: 20616314
- PMCID: PMC3050675
- DOI: 10.1161/CIRCRESAHA.110.224345
Mitochondria control functional CaV1.2 expression in smooth muscle cells of cerebral arteries
Abstract
Rationale: Physiological functions of mitochondria in contractile arterial myocytes are poorly understood. Mitochondria can uptake calcium (Ca(2+)), but intracellular Ca(2+) signals that regulate mitochondrial Ca(2+) concentration ([Ca(2+)](mito)) and physiological functions of changes in [Ca(2+)](mito) in arterial myocytes are unclear.
Objective: To identify Ca(2+) signals that regulate [Ca(2+)](mito), examine the significance of changes in [Ca(2+)](mito), and test the hypothesis that [Ca(2+)](mito) controls functional ion channel transcription in myocytes of resistance-size cerebral arteries.
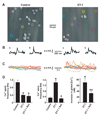
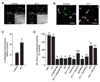
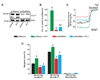
Methods and results: Endothelin (ET)-1 activated Ca(2+) waves and elevated global Ca(2+) concentration ([Ca(2+)](i)) via inositol 1,4,5-trisphosphate receptor (IP(3)R) activation. IP(3)R-mediated sarcoplasmic reticulum (SR) Ca(2+) release increased [Ca(2+)](mito) and induced mitochondrial depolarization, which stimulated mitochondrial reactive oxygen species (mitoROS) generation that elevated cytosolic ROS. In contrast, a global [Ca(2+)](i) elevation did not alter [Ca(2+)](mito), mitochondrial potential, or mitoROS generation. ET-1 stimulated nuclear translocation of nuclear factor (NF)-kappaB p50 subunit and ET-1-induced IP(3)R-mediated mitoROS elevated NF-kappaB-dependent transcriptional activity. ET-1 elevated voltage-dependent Ca(2+) (Ca(V)1.2) channel expression, leading to an increase in both pressure (myogenic tone)- and depolarization-induced vasoconstriction. Baseline Ca(V)1.2 expression and the ET-1-induced elevation in Ca(V)1.2 expression were both reduced by IP(3)R inhibition, mitochondrial electron transport chain block, antioxidant treatment, and NF-kappaB subunit knockdown, leading to vasodilation.
Conclusions: IP(3)R-mediated SR Ca(2+) release elevates [Ca(2+)](mito), which induces mitoROS generation. MitoROS activate NF-kappaB, which stimulates Ca(V)1.2 channel transcription. Thus, mitochondria sense IP(3)R-mediated SR Ca(2+) release to control NF-kappaB-dependent Ca(V)1.2 channel expression in arterial myocytes, thereby modulating arterial contractility.
Figures


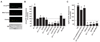


References
-
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. - PubMed
-
- Chalmers S, Olson ML, MacMillan D, Rainbow RD, McCarron JG. Ion channels in smooth muscle: regulation by the sarcoplasmic reticulum and mitochondria. Cell Calcium. 2007;42:447–466. - PubMed
-
- Poburko D, Lee CH, van Breemen C. Vascular smooth muscle mitochondria at the cross roads of Ca2+ regulation. Cell Calcium. 2004;35:509–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

