The polarization of immune cells in the tumour environment by TGFbeta
- PMID: 20616810
- PMCID: PMC3885992
- DOI: 10.1038/nri2808
The polarization of immune cells in the tumour environment by TGFbeta
Abstract
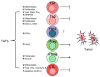
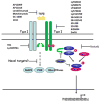
Transforming growth factor-beta (TGFbeta) is an immunosuppressive cytokine produced by tumour cells and immune cells that can polarize many components of the immune system. This Review covers the effects of TGFbeta on natural killer (NK) cells, dendritic cells, macrophages, neutrophils, CD8(+) and CD4(+) effector and regulatory T cells, and NKT cells in animal tumour models and in patients with cancer. Collectively, many recent studies favour the hypothesis that blocking TGFbeta-induced signalling in the tumour microenvironment enhances antitumour immunity and may be beneficial for cancer therapy. An overview of the current drugs and reagents available for inhibiting TGFbeta-induced signalling and their phase in clinical development is also provided.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

