Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism
- PMID: 20616882
- PMCID: PMC2899519
- DOI: 10.3389/fnene.2010.00008
Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism
Abstract
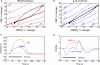
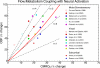
Functional magnetic resonance imaging is widely used to map patterns of brain activation based on blood oxygenation level dependent (BOLD) signal changes associated with changes in neural activity. However, because oxygenation changes depend on the relative changes in cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO(2)), a quantitative interpretation of BOLD signals, and also other functional neuroimaging signals related to blood or tissue oxygenation, is fundamentally limited until we better understand brain oxygen metabolism and how it is related to blood flow. However, the positive side of the complexity of oxygenation signals is that when combined with dynamic CBF measurements they potentially provide the best tool currently available for investigating the dynamics of CMRO(2). This review focuses on the problem of interpreting oxygenation-based signals, the challenges involved in measuring CMRO(2) in general, and what is needed to put oxygenation-based estimates of CMRO(2) on a firm foundation. The importance of developing a solid theoretical framework is emphasized, both as an essential tool for analyzing oxygenation-based multimodal measurements, and also potentially as a way to better understand the physiological phenomena themselves. The existing data, integrated within a simple theoretical framework of O(2) transport, suggests the hypothesis that an important functional role of the mismatch of CBF and CMRO(2) changes with neural activation is to prevent a fall of tissue pO(2). Future directions for better understanding brain oxygen metabolism are discussed.
Keywords: blood oxygenation level dependent; cerebral blood flow; cerebral metabolic rate of oxygen; functional magnetic resonance imaging; positron emission tomography; tissue oxygenation.
Figures

References
-
- Ances B. M., Leontiev O., Perthen J. E., Liang C., Lansing A. E., Buxton R. B. (2008). Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: implications for BOLD-fMRI. Neuroimage 39, 1510–152110.1016/j.neuroimage.2007.11.015 - DOI - PMC - PubMed
-
- Ances B. M., Liang C. L., Leontiev O., Perthen J. E., Fleisher A. S., Lansing A. E., Buxton R. B. (2009). Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum. Brain Mapp. 30, 1120–113210.1002/hbm.20574 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

