HIV gp41 engages gC1qR on CD4+ T cells to induce the expression of an NK ligand through the PIP3/H2O2 pathway
- PMID: 20617170
- PMCID: PMC2895652
- DOI: 10.1371/journal.ppat.1000975
HIV gp41 engages gC1qR on CD4+ T cells to induce the expression of an NK ligand through the PIP3/H2O2 pathway
Abstract
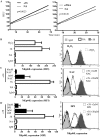
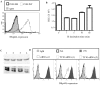
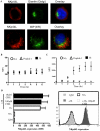
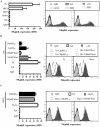
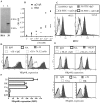
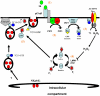
CD4(+) T cell loss is central to HIV pathogenesis. In the initial weeks post-infection, the great majority of dying cells are uninfected CD4(+) T cells. We previously showed that the 3S motif of HIV-1 gp41 induces surface expression of NKp44L, a cellular ligand for an activating NK receptor, on uninfected bystander CD4(+) T cells, rendering them susceptible to autologous NK killing. However, the mechanism of the 3S mediated NKp44L surface expression on CD4(+) T cells remains unknown. Here, using immunoprecipitation, ELISA and blocking antibodies, we demonstrate that the 3S motif of HIV-1 gp41 binds to gC1qR on CD4(+) T cells. We also show that the 3S peptide and two endogenous gC1qR ligands, C1q and HK, each trigger the translocation of pre-existing NKp44L molecules through a signaling cascade that involves sequential activation of PI3K, NADPH oxidase and p190 RhoGAP, and TC10 inactivation. The involvement of PI3K and NADPH oxidase derives from 2D PAGE experiments and the use of PIP3 and H2O2 as well as small molecule inhibitors to respectively induce and inhibit NKp44L surface expression. Using plasmid encoding wild type or mutated form of p190 RhoGAP, we show that 3S mediated NKp44L surface expression on CD4(+) T cells is dependent on p190 RhoGAP. Finally, the role of TC10 in NKp44L surface induction was demonstrated by measuring Rho protein activity following 3S stimulation and using RNA interference. Thus, our results identify gC1qR as a new receptor of HIV-gp41 and demonstrate the signaling cascade it triggers. These findings identify potential mechanisms that new therapeutic strategies could use to prevent the CD4(+) T cell depletion during HIV infection and provide further evidence of a detrimental role played by NK cells in CD4(+) T cell depletion during HIV-1 infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003;84:1649–1661. - PubMed
-
- Stevenson M. HIV-1 pathogenesis. Nat Med. 2003;9:853–860. - PubMed
-
- Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, et al. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. - PubMed
-
- Varbanov M, Espert L, Biard-Piechaczyk M. Mechanisms of CD4 T-cell depletion triggered by HIV-1 viral proteins. AIDS Rev. 2006;8:221–236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

